
- 936 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Thermal and Catalytic Processes in Petroleum Refining
About this book
This text examines the thermal and catalytic processes involved in the refining of petroleum including visbreaking, coking, pyrolysis, catalytic cracking, oligomerization, alkylation, hydrofining, hydroisomerization, hydrocracking, and catalytic reforming. It analyzes the thermodynamics, reaction mechanisms, and kinetics of each process, as well as
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
1
Thermodynamic Analysis of Technological Processes
The thermodynamic study of technological processes has two objectives:
Determination of the overall thermal effect of chemical transformations that take place in the industrial process
Determination of the equilibrium composition for a broad range of temperatures and pressures in order to deduce optimum working conditions and performances
The manner in which the two objectives are approached within the conditions of chemical technology is different from the classical approach and requires the use of the specific methodology outlined in this chapter.
1.1 Calculation of the Overall Thermal Effect
In practical conditions under which technological processes operate, the main reaction may be accompanied by secondary reactions. In many cases the transformation is of such complexity that it cannot be expressed by a reasonable number of chemical reactions.
When calculating the heat of reaction in such situations, in order to avoid the difficulties resulting from taking into account all reactions many times in the calculation, simplified approaches are taken. Thus, one may resort to the approximation of limiting the number of the reactions taken into consideration, or to take account only the main reaction. Such approximations may lead to significant errors.
Actually, the exact value of the thermal effect can be calculated without having to resort to such approximations. Since the thermal effect depends only on the initial and the final state of the system (the independence of path, as stipulated by the second principle of thermodynamics), it may be calculated based on the initial and final compositions of the system, without having to take in account the reactions that take place.
Accordingly, the classic equations, which give the thermal effect of a chemical reaction:


may be written under the form:


The heats of formation ΔHf and of combustion ΔHC for hydrocarbons and organic compounds, which are of interest in studying petrochemical processes, are given in thermodynamic data books [1,2]. The values are usually given for temperature intervals of 100 K, within which linear interpolation is accurate. Thus, the calculations that use the heat capacities may be avoided.
Example 1.1 shows how to perform the calculations by means of relations (1.3) and (1.4).
Example 1.1. Compute the overall thermal effect of an industrial dehydrogenation process of isopentane to isoprene at 600°C.
The composition of the streams at the inlet and outlet of the reactor is given in Table 1.1. The coke composition by weight, is 95% carbon and 5% hydrogen.
Table 1.1
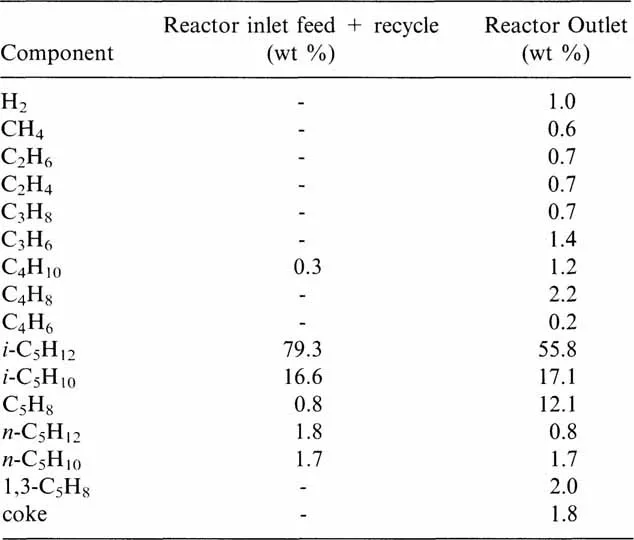
The calculations of the heat of formation at the inlet and the outlet of the reactor at 600°C are collected in Table 1.2.
Table 1.2
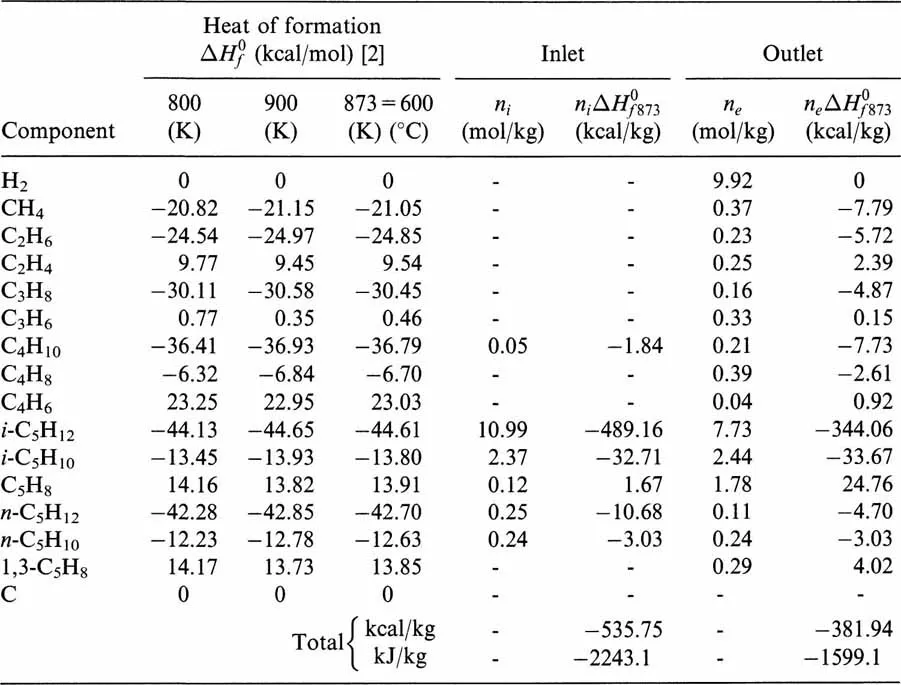
According to Eq. (1.3), the overall thermal effect per unit mass (kg) of feed will be:
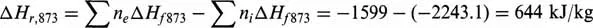
Since the process is performed at a temperature much above the critical point and at low pressure, no deviations from the ideal state have to be considered.
In many cases it is convenient to express the thermal effect on the basis of the reacted isopentane or of the formed isoprene.
For this example, according to Table 1.1, 793 − 558 = 235g, isopentane reacts and 121 − 8 = 113g, isoprene is formed. In these conditions, the thermal effect expressed per mole of reacted isopentane is:
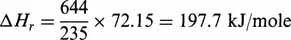
and per mole of produced isoprene:
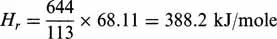
If only the main reaction:
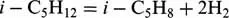
is taken into account, then according to the Eq. (1.1) one obtains:
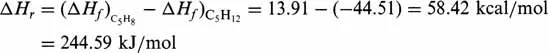
the value being the same whether expressed per mole of isopentane or of isoprene.
This example shows that large errors may result if the computation of the overall thermal effect is not based on the real compositions of the inlet and outlet streams of the reactor.
Eq. (1.4) makes it possible to compute the thermal effects by using the heats of combustion. This is useful for the conversion of petroleum fractions of other feedstocks consisting of unknown components. In such cases it is usually more convenient to perform the calculation in weight units, by modifying the terms n and AH accordingly.
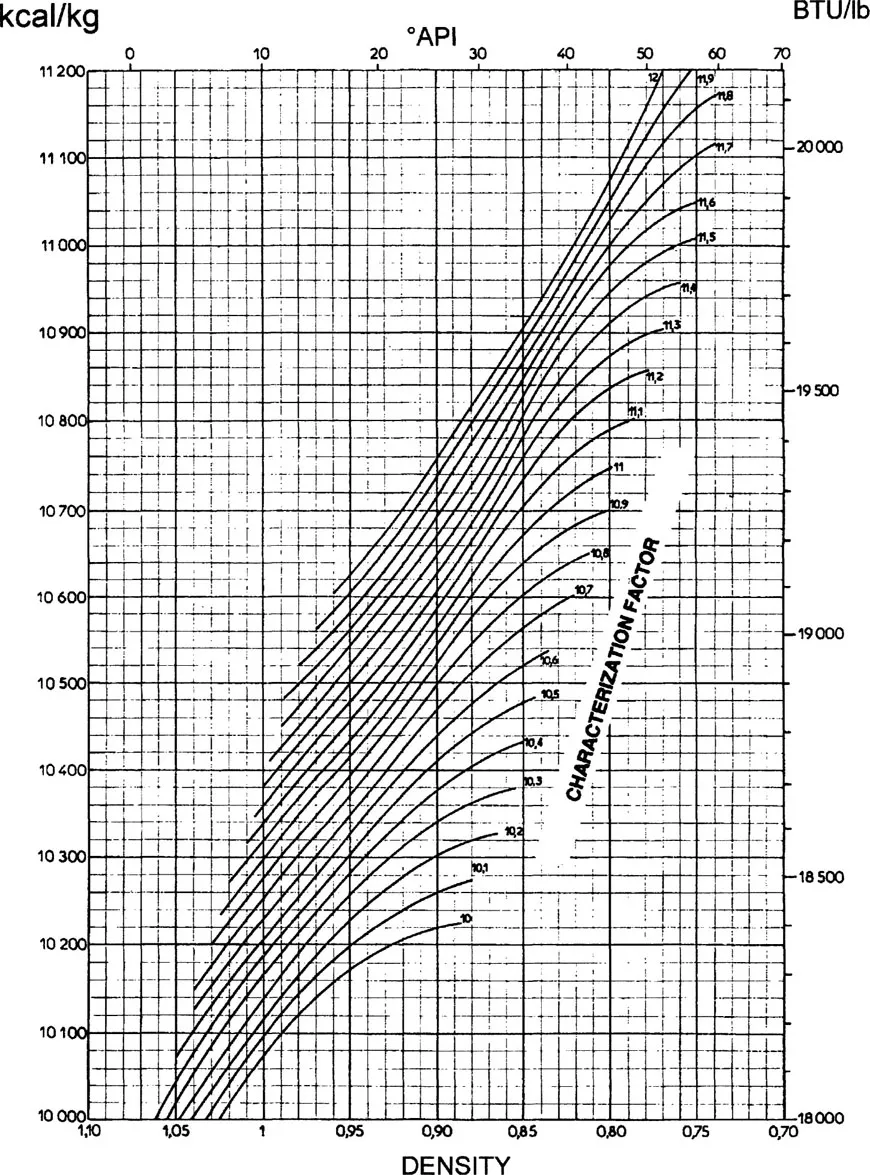
For liquid petroleum fractions, the heats of combustion may be determined by using the graph of Figure 1.1 [3], from the known values of the specific gravity and the characterization factor.
Figure 1.1 Heat of combustion of petroleum fractions. Final state: gaseous CO2 and liquid water at 15°C.
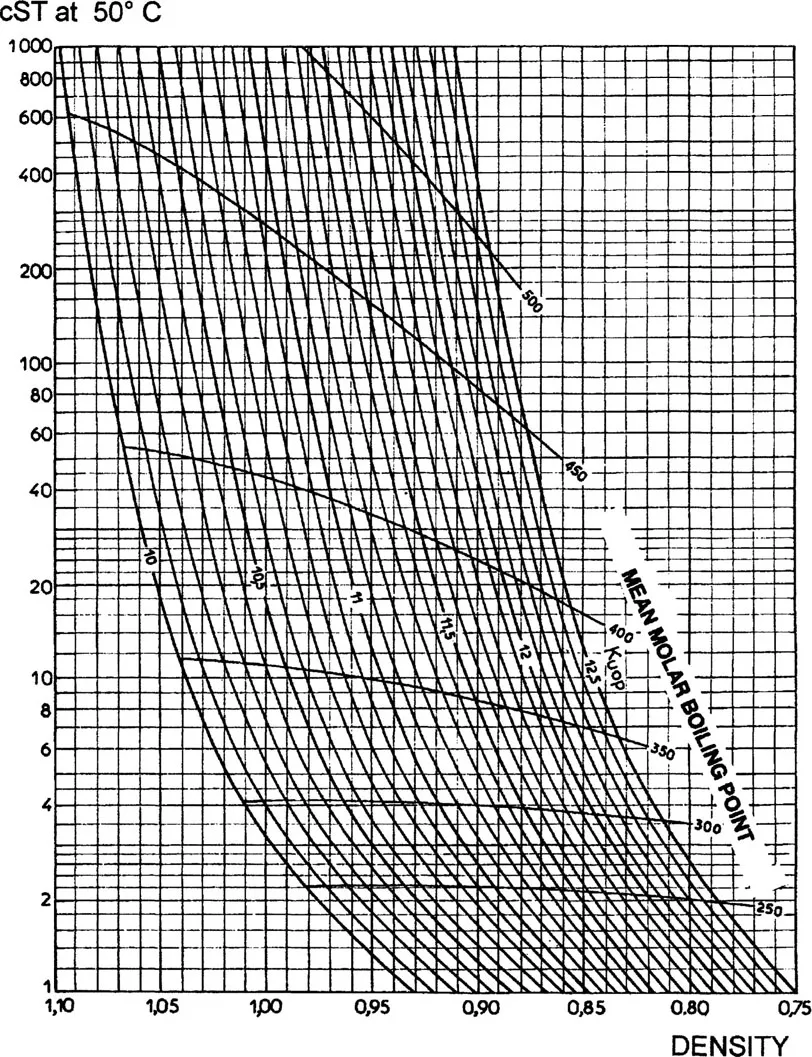
The characterization factor of residues may be determined graphically from the viscosity, by means of Figure 1.2 [3].
Figure 1.2 KUOP as function of the kinematic viscosity and density.
The heat of combustion of coke is determined experimentally or less precisely on the basis of the elementary composition.
The heats of combustion of gaseous components may be found in data books [1,2], or may be calculated from the heats of formation [2], by applying Eq. (1.1). For hydrocarbons, this equation takes the form:
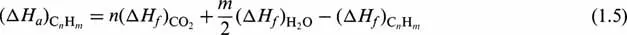
This heat of combustion of gases must be brought to the same reference state as that of liquid fractions, i.e. 15°C and liquid water. For these conditions, Eq. (1.5) becomes:
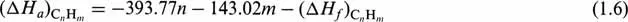
It must be noted that Eq. (1.6) gives the heat of combustion in thermodynamic notation, expressed in kJ/mole. ...
Table of contents
- Cover
- Halftitle
- Title
- Copyright
- Contents
- Preface
- Preface to the Romanian Edition
- PART I. Thermal Conversion Processes
- PART II. Processes on Acid Catalysts
- PART III. Processes on Metallic Catalysts
- PART IV. Processes using Bifunctional Catalysts
- Appendix Influence of tlze nli-Alkanes Ratio in the Pyroljvis Feed on the EthenelPropene Ratio in the Products
- Index
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Thermal and Catalytic Processes in Petroleum Refining by Serge Raseev in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Chemistry. We have over one million books available in our catalogue for you to explore.