
eBook - ePub
Bioassay Techniques for Drug Development
- 240 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Bioassay Techniques for Drug Development
About this book
The goal of an activity-directed isolation process is to isolate bioactive compounds which may provide structural leads of therapeutic importance. Whereas the traditional process of drug development is long and expensive, simple and rapid bioassays can serve as the starting point for drug discovery. This book presents a range of "bench top" bioassa
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
1.0 GENERAL INTRODUCTION
Extracts from natural product sources have served as a valuable source of molecular diversity in many drug discovery programs, and several important drugs have been isolated from natural products. In any natural product isolation program in which the end-product is to be a drug or a lead compound, some type of bioassay screening or pharmacological evaluation must necessarily be used to guide the isolation process towards the pure bioactive component.
The pharmacological evaluation of extracts of organisms and pure isolates is an essential aspect of the drug discovery process and developments in the area of in vitro techniques have substantially transformed this facet of natural product chemistry. While it previously took weeks or even months to test a sample for some assays, it can now take only a few hours.
One should also distinguish between the “primary bioassay screens” from “secondary screens”. Primary bioassays are assays which can be rapidly applied to a large number of samples to determine if any bioactivity of the desired type is present. They should therefore have high capacity, low cost and provide the results quickly. They need not be quantitative. “Secondary testing” procedures involve more detailed testing of lead compounds on a number of model systems in order to select compounds for clinical trials. They are usually low capacity, slow and costly assays.
The main requirements which a primary bioassay screen should meet are the following:
- The bioassay results should predict some type of therapeutic potential, either directly or by analogy with clinically effective drugs which have also been screened by the same procedure.
- Potentially useful pharmacological activity should not go undetected even though the activity may be either unexpected or unique.
- The probable nature of the activity should be indicated so that subsequent research can be organized intelligently.
- The primary bioassay screening test should be tolerant of the many impurities present in a crude extract and yet it should be sensitive enough to reveal presence the potentially interesting substances present in low concentrations (levels of about 0.0001% of an active compound in an extract, based on the dried weight of the extracted organism, should be detectable).
- The bioassay procedure should be unbiased and it should allow for the coding of all samples, including both “known” reference materials (standards) and “unknown” test samples.
- The results obtained should be reproducible.
- The screen should allow the use of both crude materials and pure isolates so that the procedure can be used to direct the extraction, isolation and purification work of the natural product chemist.
- Completion of a single bioassay screen should not require more than 1.0–2.0 g of the crude dry natural material (plant or animal extract).
- The primary bioassay screen should have a high throughput, even if the information content is low, with the results becoming available quickly.
- The procedure should not require expensive equipment or a sophisticated laboratory environment so that the primary level screening experiment can be conducted synchronously with the fractionation process.
- The procedure should be compatible with the use of dimethyl sulfoxide (DMSO) since DMSO is commonly employed to solubilize extracts or pure polar compounds for screening.
- The procedure should be simple enough to be taught easily to laboratory technicians so that highly trained and qualified researchers are not required for the routine operation of the bioassay program.
- The test animals (if required for the bioassay) should be easily obtainable, easily handled, easily bred and resistant to infections.
(13) Finally the bioassay should be economical to conduct over extended time periods.
A hit rate of 1% or less is generally considered a reasonable and one then proceeds from primary screening to secondary screening for profile selectivity and in order to establish biological activity.
The bioassay-guided natural product drug discovery research can be broadly divided into four approaches:
- the use of a single bioassay technique in order to search for a specific type of pharmacological activity (such as antidiabetic, cardiotonic or antiinflammatory activity);
- the use of a battery of specific bioassay techniques with each procedure directed to discover a different type of useful activity;
- the use of a single bioassay technique designed to detect multiple activities (non-specific bioassay). For example cytotoxicity bioassays can be employed to predict a variety of biological activities such as antitumor, insecticidal and antimicrobial activities. Another example of this approach is the study of drug-induced symptomatology (CNS depressant, tranquilizing, psychotropic, skeletal muscle relaxant, sympathetic stimulant, diuretic, metabolic poison, vasodilatory etc.) after a single intraperitoneal injection of an extract to an intact anaesthetized rat;
- the use of a combination of a variety of bioassays in order to detect specific activities as well as to detect multiple activities.
The choice of the screening approach to be adopted generally depends on the target disease as well as on the available information about the target organism (plant, marine animal, etc.) to be studied. For example if a plant has a ethanopharmacological history of use against a particular disease, then one would logically use a specific bioassay technique (single goal screening) which can predict the reputed therapeutic activity in order to isolate the lead which is responsible for that biological activity. Similarly, based on the chemotaxonomic knowledge of related species, one can select one or more bioassay screens based on the reputed or reported bioactivity (or use) of related species. Bio-rational selection is based on the knowledge of plants and animals and their behavior in certain circumstances. For example some primates may eat certain types of grasses in cases of indigestion. The zoo-pharmacognosic knowledge can hence help in the selection of specific bioassays. Similarly certain plants many exhibit resistance against insect attack. They can therefore be screened for insecticidal compounds by using pesticidal bioassays. In the case of random or blind collection of common, unusual or uninvestigated organisms, it is better to use a battery of bioassay screens at the extract level and follow the most prominent activity subsequently through a specific bioassay technique (Scheme-1). A typical flow diagram of a bioassay-guided isolation of bioactive isolates from natural sources (plants, microorganisms, marine animals, etc.) is presented in (Scheme-2).
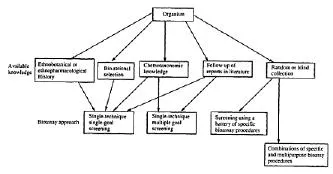
Scheme-1:Approaches to bioactivity-directed natural poduct drug development.
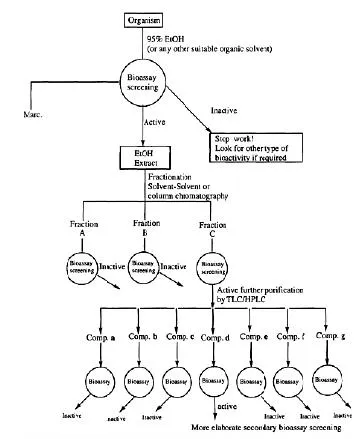
Scheme-2: Bioactivity-directed isolation of natural products.
PART-A Bench-Top and Primary Bioassay Screening
1.1 TOXICITY ASSAYS
1.1.1 Brine-Shrimp Lethality Assay
Bioactive compounds are often toxic to shrimp larvae. Hence, in vivo lethality to shrimp larvae can be used as a rapid and simple preliminary monitor for bioactive compounds during the isolation of natural products. The eggs of the brine shrimp Artemia salina (Leach) are readily available as fish food in pet shops. When placed in artificial sea water, the eggs hatch within 48 hours, providing large numbers of larvae. These tiny shrimp larvae have been extensively used as a tool to monitor the cytotoxicity of samples under study. This is a rapid, inexpensive, in-house, general bioassay which has been developed for screening, fractionation and monitoring of physiologically active natural products (Meyer et al., 1982).
Materials
- Artemia salina Leach (brine shrimp eggs)*
- Sea salt+
- Small tank with perforated dividing dam and cover to grow shrimps; lamp to attract shrimps
- Syringes; 5.0 ml, 0.5 ml, 100 μl and 10 μl
- 2 dram vials (9 per sample+1 control)
- Magnifying glass
- Organic solvents (methanol, dichloromethane, chloroform, DMSO etc.)
- Distd. water
- Pasteur pipettes
- Aluminium foil
- Test sample (crude extract of organism, pure natural product or synthetic compound)
The steps involved in the Brine-shrimp lethality assay are as follows:
- Artificial “sea water” is prepared by dissolving ca. 3.8 g sea salt per liter of water and filtered.
- “Sea water” is placed in a small unequally divided tank and shrimp eggs added to the larger compartment of the tank which is darkened by covering it with aluminium foil. The illuminated compartment attracts shrimp larvae (nauplii) through perforations in the dam.
- Allow 2 days at room temperature (22–29°C) for the shrimps to hatch and mature.
- Prepare vials for testing; for each fraction, test initially at 1000, 100, and 10 μg/ml; prepare 3 replicates for each concentration making a total of 9 vials; weigh 20 mg of sample and add 2 ml of organic solvent (20 mg/2 ml); from this solution transfer 500, 50, or 5 μl to vials corresponding to 1000, 100, or 10 μg/ml, respectively. Evaporate solvent under nitrogen and then place under high vacuum for about 30 min; the volatile organic solvents will evaporate overnight. Alternatively, polar insoluble materials may be dissolved in DMSO, and upto 50 μl may be added per 5 ml of “sea water” before DMSO toxicity affects the results.
- After 2 days (when the brine shrimp larvae have matured), add 5 ml “sea water” to each vial and add 10 shrimps per vial with the help of Pasteur pipette (30 shrimps per dilution). The vials are maintained under illumination.
- After 24 hours have elapsed, count and record the number of surviving shrimps, with the aid of a 3×magnifying glass.
- Analyze data with a Finney computer program (Probit analysis) to determine LC50 values and 95% confidence intervals.**
- Additional dilutions of less than 10 μg/ml may be needed for potent materials. Intermediate concentrations can be prepared and tested to narrow the confidence intervals.
1.1.2 Brine-Shrimp Microwell Cytotoxicity Assay
A new microplate assay for cytotoxicity or lethality determination using brine-shrimp (Artemia salina) has been developed which gives results comparable to the vial method described under the heading of brine-shrimp lethality assay (Section 1.1.1). The assay reliably correlates with KB cell toxicity assays and thus provides a convenient means by which
the presence of cytotoxic natural products may be detected during the fractionation and isolation of natural products.
Materials
- Brine shrimp eggs (Artemia salina)
- Sea salt
- Dried yeast
- 96-Well microplates
- DMSO
- Pasteur pipette
- Binocular microscope (10.30×)
- Methanol
- Incubator
- Beaker
- Test sample (plant extract, pure natural product or synthetic compound)
Brine-shrimp microwill cytotoxicity assay typically consists of the following assay steps:
- Artificial sea water is prepared by dissolving sea salt in distd. water (40 g/lit.) supplemented with 6 mg/lit. dried yeast.
- Brine shrimp eggs (Artemia salina) are hatched in artificial sea water during 48 hours incubation in a warm room (22–29°C).
- Brine shrimp larvae (nauplii) are collected with a Pasteur pipette after attracting the organisms to one side of the vessel with a light source. Nauplii are separated from the eggs by pipetting them 2–3 times in small beakers containing sea water.
- The test sample (20 mg of crude extracts or 4 mg for pure compound) is made up to 1 mg/ml in artificial sea water (water insoluble compounds or extracts can be dissolved in 5 ml DMSO prior to adding sea water).
- Serial dilutions are made in wells of 96-well microplates in triplicate in 100 μl sea water.
7. A suspension of nauplii containing 10–15 brine shrimp larvae (100 ml) are added to each well with the help of a Pasteur pipette and the covered microwell plate incubated at 22–29°C for 24 hours.
8. The plates are then examined under a binocular microscope (12.5×) and the number of dead (non-mobile) nauplii in each well counted.
9. 100 μl methanol is then added to each well and after 15 minutes the total number of shrimps in each well is counted.
10. LC50 values are then calculated by using Probit analysis (“FIN” program).
1.1.3 Crown Gall Tumor Inhibition Assay (Potato Disc Antitumor Assay)
Crown gall is a neoplastic disease of plants which is induced by the gram negative bacteria Agrobacterium tumefaciens. The bacteria possess large Ti (tumor inducing) plasmids which carry genetic information (T DNA) that transform normal, wounded, plant cells into autonomous tumor cells. Since the mechanism of tumor induction is similar to that in animals, this test system has been used to evaluate and pre-screen the an...
Table of contents
- COVER PAGE
- TITLE PAGE
- COPYRIGHT PAGE
- PREFACE
- 1.0 GENERAL INTRODUCTION
- PART-A BENCH-TOP AND PRIMARY BIOASSAY SCREENING
- PART-B HIGH-THROUGHPUT SCREENING
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Bioassay Techniques for Drug Development by Atta-ur-Rahman,M. Iqbal Choudhary,William J. Thomsen in PDF and/or ePUB format, as well as other popular books in Medicine & Pharmacology. We have over one million books available in our catalogue for you to explore.