
- 246 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Fire Investigation
About this book
Fire Investigation covers the concepts and theories used to determine a specfic fire has been deliberately or accidentally set. The author clearly explains the concepts needed to gain insight into a fire scene investigation, including the dynamics of the fire, the necessary conditions for a fire to start and be maintained, the different types of co
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
1
An introduction to fires and fire investigation
Niamh Nic Daéid
Introduction
Fire investigation can occur in two different stages. The first involves examination of the fire scene to determine the cause, origin and development/spread of fire. The second involves laboratory analysis of samples recovered from a fire scene normally when arson is suspected. While both of these may be linked together, they may be activities carried out by different personnel with different backgrounds and experience.
Scene investigation
In order to successfully carry out fire scene investigations, the investigator should have an understanding of a variety of concepts. These include:
- the fundamental practices and methodology involved in fire scene and crime scene investigation;
- the necessary conditions for a fire to be initiated and maintained;
- knowledge of the dynamics of a fire and factors influencing fire development and spread;
- knowledge of different types of fuel packages, their auto-ignition temperatures, behaviour in fires and the level of heat release which they may produce;
- different types of burn and smoke patterns and their interpretation;
- sampling protocols, packaging, etc.
Only with a sound knowledge of these and other factors can an investigator carry out his or her scene investigation efficiently and correctly.
Laboratory analysis
Laboratory based analysis requires the appropriate skill and knowledge of relevant scientific instrumentation, proper laboratory practice in dealing with crime scene evidence and an understanding of the nature of materials including flammable liquids, their pyrolysis and combustion products as well as an ability to interpret the results of their analysis.
This chapter acts as a summary of the phenomena of combustion, the development of fires in compartments and the spread of fire from one compartment to the next. Reference has been made liberally to texts such as Kirk’s Fire Investigation [1] and An Introduction to Fire dynamics [2] and the reader is referred to these texts for a fuller explanation.
Types of fires
The determination of the type of fire which has occurred often falls to either fire brigade personnel, police officers, scenes of crime officers, forensic scientists or private fire investigators who specialise in fire scene investigation. Deliberate fires or arsons are estimated to account for between 50% and 60% of fires in some parts of the UK [3], with direct financial costs of billions of pounds per annum. Over the last 10 years the number of arson fires in the UK has increased by over 40% and arson fires in vehicles have tripled. The average national detection rate of arson fires remains low at 8% in 2002 (for England and Wales) [4]. Motivations for arson are often varied and complex and can include:
- criminal intent such as covering up other crimes (theft, murder);
- financial gain (insurance claims);
- civil disorder (youth disorder, vandalism);
- malicious intent (grudge/reprisal against a particular race, religion, societal group);
- as part of a series of crimes of a known arsonist;
- acts of terrorism with motivations such as urban unrest, racial or religious hatred or for political reasons.
Not all fires are deliberately set and many arise from various types of accidental cause. This can include spontaneous combustion of materials, careless discarding of smoking materials, careless use of candles, electrical faults and so on. Many of these causes are dealt with in Chapters 2 and 3.
Fire investigators
Because of the differing nature of fire scenes, a number of interested parties can be involved. These may include the fire brigade and brigade fire investigation units, the police and assorted support team (forensic scientists, scenes of crime officers, etc.), insurance agents and loss adjusters, various independent fire investigators, and representatives of relevant local authorities in relation to public health and/or safety. Each team on site may wish to carry out an investigation and prepare a report. In the UK the recent Arson Scoping Study [6] has recommended that agencies such as those mentioned should strive to work co-operatively (where practical) in an effort to attempt to reduce the incidents of fire.
Fire and combustion
Fire can be defined as an exothermic chemical reaction involving the oxidation of some substance (a fuel) resulting in the release of energy in the form of light and heat. The conditions necessary for combustion to occur are the presence of a fuel, a source of oxygen (air) and heat. There is also the requirement, in most cases, for an initial ignition source to provide sufficient energy to overcome any energy barriers and ignite the fuel present.
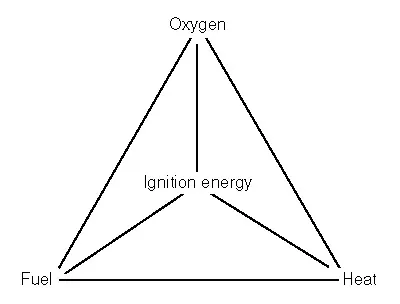
Figure 1.1 The fire quadrangle
The presence of fuel, oxygen, heat and an appropriate ignition source of sufficient energy comprise the fire diamond or quadrangle (Figure 1.1). Once the fire has been established, fuel, oxygen and heat alone comprise the fire triangle and removal of any of these three conditions results in the suppression of the fire.
Fire is a series of exothermic oxidative reactions involving the fuels present. Most fuels will contain hydrogen and carbon, and water and carbon dioxide are the major products of combustion with other products including carbon monoxide and oxides of sulphur, nitrogen and other compounds which may be present and which contribute to smoke.
Types of Fuel
Most fires will involve combustible solids, though liquid and gaseous fuels are also found. The range of fuels involved is very large and encompasses simple hydrocarbon gases to chemically complex solids, either natural, synthetic or semi-synthetic. These fuels will burn under appropriate conditions, reacting with oxygen and releasing heat and combustion products. Flame itself is a gas phase phenomenon and flaming combustion of solids and liquids require their initial conversion to a gaseous state. For liquids this is relatively straightforward through boiling at the surface. Solids, unless they sublime, must undergo a chemical decomposition process called pyrolysis to produce sufficient low molecular weight volatile components which can enter the gaseous phase. In order to achieve this, a high degree of energy is required and as a consequence the temperature at the surface of burning solids tends to be quite high.
Some relevant properties of materials
Flammability (explosive) limits/range
Mixtures of flammable gases or vapours with air will combust only when they are within particular ranges of gas–air concentration. Outside of these limits the fuel–air mixture is either too lean or too rich to ignite. If the fuel–air mixture is confined in a closed system then the mixture must explode to ignite and the explosive limits and flammability limits are
Table 1.1 Flammability limits of common gases and liquids (adapted from reference [2])
the same. In an open system other factors such as temperature of the surrounding medium will effect the flammability limits. Table 1.1 illustrates some flammability (explosive) limits of common gases and liquids.
Vapour density [2]
This is a property of a vapour that predicts its behaviour when released in air. It is defined as the density of the vapour relative to the density of air and is calculated by dividing the molecular weight of the gas by that of air (approx. 29). A gas with vapour density greater than 1 is heavier than air and will tend to settle through the air into which it is released until it encounters an obstruction, when it will tend to spread out at this level. A similar situation exists with a gas of vapour density less than one which will spread out at ceiling level. Inevitably there will be some mixing of the gas with air due to diffusion, the degree of which will depend on the difference bet...
Table of contents
- Cover Page
- Title Page
- Copyright Page
- Contributors
- Preface
- 1 An introduction to fires and fire investigation
- 2 Fires from causes other than electrical malfunctions
- 3 Electricity and fire
- 4 The use of laboratory reconstruction in fire investigation
- 5 Modern laboratory techniques involved in the analysis of fire debris samples
- 6 Interpretation of laboratory data
- 7 Sources of interference in fire debris analysis
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Fire Investigation by Niamh Nic Daeid, James R. Robertson in PDF and/or ePUB format, as well as other popular books in Law & Forensic Science. We have over one million books available in our catalogue for you to explore.