
Trends in Quorum Sensing and Quorum Quenching
New Perspectives and Applications
- 390 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Trends in Quorum Sensing and Quorum Quenching
New Perspectives and Applications
About this book
The book on Trends in Quorum Sensing and Quorum Quenching: New Perspectives and Applications focuses on the recent advances in the field of quorum sensing in bacteria and the novel strategies developed for quorum sensing inhibition. The topics covered are multidisciplinary and wide-ranging,and includes quorum sensing phenomenon in pathogenic bacteria, food spoilers, and agriculturally relevant bacteria. The applications of quorum sensing inhibitors such as small molecules, bioactives, natural compounds, and quorum quenching enzymes in controlling bacterial infections in clinical settings, agriculture and aquaculture are discussed. The potential use of quorum quenching enzymes for mitigating biofouling is also covered. Special focus is given to exploring quorum sensing inhibitors from microbes and flora inhabiting biodiversity rich regions including tropical rain forests and marine environments.
Key features:
- Covers the fundamental aspects, the progress and challenges in the field of quorum sensing and quorum quenching
- Reviews quorum sensing in Gram-positive and Gram-negative bacteria of clinical, agricultural, and industrial relevance
- Discusses the application and future trends of quorum sensing inhibitors from lab to clinical and environmental settings
- Provides comprehensive coverage on molecular mechanisms in bacterial signaling
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
1
Expanding Roles and Regulatory Networks of LadS/RetS in Pseudomonas aeruginosa
Chuanmin Zhou, Maryam Dadashi, and Min Wu
1.1 The Two-Component Systems
1.2 Discovery of RetS and LadS
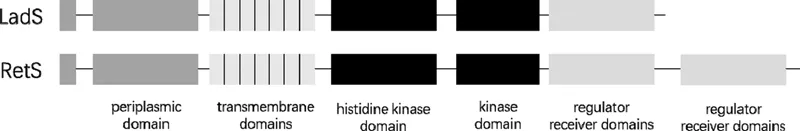
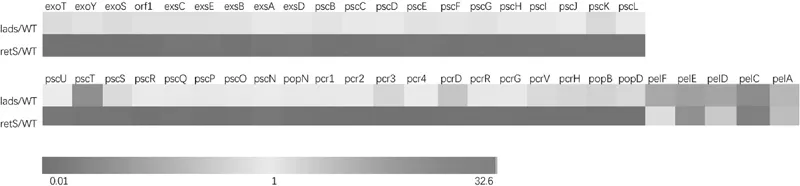
1.3 Opposing Roles of RetS and LadS
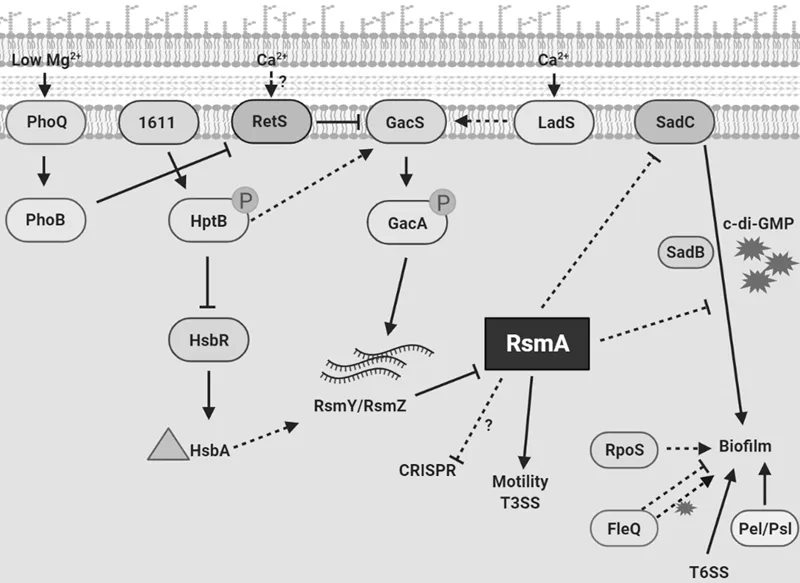
1.4 Function of LadS/RetS Is Dependent on Small RNAs
1.5 Other Hybrid Sensor Kinases in Gac/Rsm Pathway
Table of contents
- Cover
- Half Title
- Title Page
- Copyright Page
- Table of Contents
- Preface
- Editors
- Contributors
- 1. Expanding Roles and Regulatory Networks of LadS/RetS in Pseudomonas aeruginosa
- 2. Autoinducer-1 Quorum Sensing Communication Mechanism in Gram-Negative Bacteria
- 3. Toward a Systematic Genomic Survey of Bacterial Quorum Sensing Genes: Cross Cutting Regulatory and Genomic Concepts
- 4. Old Acquaintances in a New Role: Regulation of Bacterial Communication Systems by Fatty Acids
- 5. Analysis of Quorum Sensing by Surface-Enhanced Raman Scattering Spectroscopy
- 6. Quorum Sensing in Pseudomonas aeruginosa: From Gene and Metabolic Networks to Bacterial Pathogenesis
- 7. Quorum Sensing in Vibrios
- 8. Quorum Sensing in Lactic Acid Bacteria
- 9. Role of N-acyl-homoserine Lactone QS Signals in Bacteria-Plant Interactions
- 10. Quorum Sensing and the Environment: Open Questions in Plant-Associated Bacteria
- 11. In Silico Mining of Quorum Sensing Genes in Genomes and Metagenomes for Ecological and Evolutionary Studies
- 12. Bacterial Quorum Sensing in Multispecies Communities: The Presence, Functions and Applications
- 13. Breaking Bad: Understanding How Bacterial Communication Regulates Biofilm-Related Oral Diseases
- 14. Quorum Sensing in Autotrophic Nitrogen Removal Systems for Wastewater Treatment
- 15. Mechanism and Types of Quorum Sensing Inhibitors
- 16. Role of Small Volatile Signaling Molecules in the Regulation of Bacterial Antibiotic Resistance and Quorum Sensing Systems
- 17. Recent Advances in Science of Quorum Sensing: An Overview of Natural Product Inhibitors
- 18. Nanomaterials as Quorum Sensing Inhibitors
- 19. Phytochemical Compounds Targeting the Quorum Sensing System as a Tool to Reduce the Virulence Factors of Food Pathogenic Bacteria
- 20. Targeting Bacterial Communication to Improve Bacterial Infections Therapy: Implications for Phage-Based Treatments—A Mathematical Perspective
- 21. Quorum Quenching Monoclonal Antibodies for the Detection and Treatment of Gram-Negative Bacterial Infections
- 22. Novel Intervention Techniques in the Food Industry
- 23. Quorum Sensing and Quorum Quenching in Food-Related Bacteria
- 24. Quorum Quenching as an Anti-biofouling Strategy for Wastewater Reuse and Biofouling Affected Industries
- 25. Application of Quorum Sensing Inhibitors in Anti-biofouling Membranes
- 26. Quorum Sensing and Quorum Quenching Based Antifouling Mechanism: A Paradigm Shift for Biofouling Mitigation in a Membrane Bioreactor (MBR)
- 27. Application of Quorum Quenching in the Control of Animal Bacterial Pathogens
- Index
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app