
- 424 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Signaling Through Cell Adhesion Molecules
About this book
The field of signal transduction research is one of the fastest growing in all of biomedical research in recent years. Signaling through cell adhesion molecules have long been of interest because of their importance in embryonic development, homeostasis, immune responses, wound healing , and malignant transformation. However, it is only recently re
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
SECTION I
Protein-Protein Interactions and Early Events in Integrin Signaling
Chapter The Use of Chimeric Receptors in the Study of Integrin Signaling | 1 |
Contents
I. | Introduction |
II. | Activation of Tyrosine Phosphorylation |
A. Overview | |
B. Protocols | |
C. Materials | |
D. Buffers | |
III. | Inhibition of Cell Attachment |
A. Overview | |
B. Protocols | |
C. Materials | |
IV. | Inhibition of Cell Spreading |
A. Overview | |
B. Protocols | |
C. Materials | |
D. Buffers | |
V. | Concluding Remarks |
Acknowledgments | |
References | |
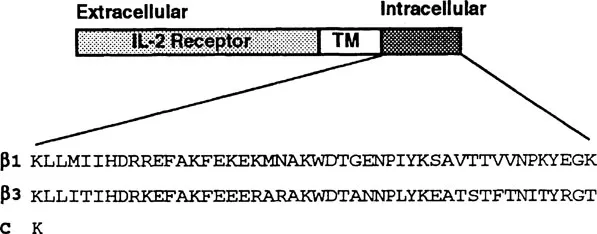
FIGURE 1
Chimeric receptors. Chimeric receptors consist of the extracellular and transmembrane ™ domains of the tac subunit of the IL-2 receptor32 connected to various integrin β cytoplasmic domains. The amino acid sequences of the wild-type β1 and β3 cytoplasmic domains are shown. The control receptor (C) consists of the extracellular and transmembrane domains of the IL-2 receptor connected to an intracellular lysine (K) residue.
Chimeric receptors. Chimeric receptors consist of the extracellular and transmembrane ™ domains of the tac subunit of the IL-2 receptor32 connected to various integrin β cytoplasmic domains. The amino acid sequences of the wild-type β1 and β3 cytoplasmic domains are shown. The control receptor (C) consists of the extracellular and transmembrane domains of the IL-2 receptor connected to an intracellular lysine (K) residue.
I. Introduction
Integrins mediate the bidirectional transfer of signals across the plasma membrane. These signals regulate cell adhesion as well as adhesion-dependent aspects of cell behavior including cell proliferation, survival, and differentiation.1,2,3 Integrin β cytoplasmic domains function in all steps of the adhesion process, including cell attachment, cell spreading, the formation of focal adhesions, and cell migration.4,5 They are thought to function in these processes by interacting with specific cytoplasmic proteins, thereby connecting integrins with the cell’s cytoskeletal and signal transduction systems. Although several cytoplasmic proteins have been demonstrated to bind to β cytoplasmic domains, their roles in regulating integrin function have not yet been clearly defined.2,5
To study the function of β cytoplasmic tails and the molecular mechanisms involved, we constructed chimeric receptors containing wild-type and mutant integrin β subunit cytoplasmic tails connected to the extracellular and transmembrane domain of the human interleukin-2 (IL-2) receptor which functions merely as a reporter domain (Figure 1). Clustering these chimeric receptors on the cell surface can activate signaling pathways by mechanisms similar to endogenous integrins.6 Therefore, these chimeric receptors can be used to identify and analyze signaling events triggered by integrin β cytoplasmic domains. Additionally, these chimeric receptors can function as dominant inhibitors of endogenous integrin function in a variety of processes including cell attachment and spreading, fibronectin matrix assembly, fitronectin-mediated phagocytosis, and high-affinity ligand binding.7,8,9,10,11 Identifying the mechanisms and protein interactions involved in these dominant negative effects will provide important insights into the role of β cytoplasmic domains in regulating integrin function.
Recently, we have focused our attention on defining the molecular mechanisms by which these chimeric receptors, when clustered on the cell surface, induce the tyrosine phosphorylation of specific signaling proteins, as well as the mechanisms by which these chimeric receptors inhibit cell attachment and cell spreading. In this chapter, we describe experimental protocols for these studies and provide suggestions on how these protocols can be further utilized to define mechanisms that regulate integrin function.
II. Activation of Tyrosine Phosphorylation
A. Overview
One of the first intracellular signals to be observed upon integrin clustering or integrin-mediated cell adhesion is an increase in the tyrosine phosphorylation of the focal adhesion kinase (FAK), which is a cytoplasmic tyrosine kinase.12,13 Chimeric receptors have been useful in defining a role for integra β cytoplasmic domains in triggering FAK phosphorylation, as well as in identifying amino acid motifs within p cytoplasmic domains required to activate FAK phosphorylation.6,8,14
The ability of wild-type and mutant β cytoplasmic domains to activate FAK phosphorylation is assayed by transiently transfecting normal human fibroblasts* by electroporation with plasmid DNAs encoding chimeric receptors whose expression is driven by the strong cytomegalovirus promoter. Approximately 15 to 36 h after transfection, the cells are removed from the tissue culture dishes and the chimeric receptors are clustered on the cell surface using magnetic beads coated with antibodies to the IL-2 receptor. The positively expressing cells are recovered using a magnet and then lysed. The tyrosine phosphorylation of FAK in the lysates is then assayed by Western blotting for phosphotyrosine or by immunoprecipitation of FAK, followed by Western blotting for tyrosine phosphorylation. This experimental approach is likely to be useful in testing the ability of β cytoplasmic domains to activate other integrin-triggered events. We have recently observed that the integrin P cytoplasmic domain is also sufficient to induce the tyrosine phosphorylation of p130Cas.** An example of the ability of chimeric receptors to trigger tyrosine phosphorylation is provided in Figure 2.
B. Protocols
1. Transient transfection — The transient transfection protocol for normal human fibroblasts is a three-day procedure adapted from a previously published protocol.15,16 On day 1, confluent cultures are split 1:5. On day 2, in the late afternoon, thymidine is added to a final concentration of 5.6 mM, and the cells are incubated for 10 to 18 h at 37°C to arrest them at the Gl/S boundary. The morning of day 3, the thymidine block is removed by aspirating the culture medium and rinsing the cells once with phosphate-buffered saline, pH 7.2 (PBS), and then adding fresh culture medium. The cells are incubated at 37°C for approximately 8.5 h.* The cells are then removed from the tissue culture dishes by incubation with 0.05% trypsin and 0.5 mM EDTA in Hanks balanced salt solution without Ca2+ and Mg2+, washed once with PBS and once with electroporation buffer. The cells are then resuspended at 1.5 × 106 cells per 0.5 ml of electroporation buffer. Plasmid DNA** (20 to 30 μg) is added to the cells, and the mixture is transferred to electroporation cuvettes and electroporated at 170 mV and 960 μF.*** The electroporated cells are then incubated in the cuvettes for 5 min at ambient temperature to allow the cells to recover. The cells are then transferred to 15 ml conical centrifuge tubes and washed once with culture medium. The cells are then cultured for 14 to 16 h at 37°C in medium containing 5 mM sodium butyrate to enhance the expression of the IL-2 receptor chimeras from the cytomegalovirus promoter.15 Signaling experiments are performed from 15 to 36 h after transfection.

FIGURE 2
Chimeric receptors trigger tyrosine phosphorylation of cytoplasmic proteins. Panel A. Cell lysates were generated from cells transiently transfected with either the control receptor lacking an intracellular domain © or chimeric receptors containing the β1 cytoplasmic domain. The lysates were separated by SDS PAGE, transferred to nitrocellulose and then probed with antibodies to phosphotyrosine. Panel B. The filter was stripped and then reprobed with antibodies to FAK. The position of FAK is indicated with an arrow.
Chimeric receptors trigger tyrosine phosphorylation of cytoplasmic proteins. Panel A. Cell lysates were generated from cells transiently transfected with either the control receptor lacking an intracellular domain © or chimeric receptors containing the β1 cytoplasmic domain. The lysates were separated by SDS PAGE, transferred to nitrocellulose and then probed with antibodies to phosphotyrosine. Panel B. The filter was stripped and then reprobed with antibodies to FAK. The position of FAK is indicated with an arrow.
2. Loading the magnetic beads with antibodies to the IL-2 receptor — The magnetic sorting of positively transfected cells was performed by a modification of a previously described protocol.17 The first step in this procedure is to load magnetic beads with antibodies to the IL-2 receptor. In most signaling experiments, we pool cells from 5 transfections. To magnetically sort this many cells requires 800 μl of beads which are first washed several times in 10 ml of PBS to remove the sodium azide (used as a preservative). After each wash, the beads are recovered using a magnet. The beads are then incubated with 10 μg of antibodies to the IL-2 receptor (7G7B6) in 1 ml of PBS at 37°C for 30 min. The beads are washed twice with PBS to remove the unbound antibody, once with sorting medium, and then resuspended in 5 ml of sorting medium.
3. Clustering the chimeric receptors on the cell surface — Transiently transfected normal human fibroblasts are harvested with trypsin-EDTA. Once cells are d...
Table of contents
- Cover
- Half Title
- Title Page
- Copyright Page
- Table of Contents
- Section I: Protein-Protein Interactions and Early Events in Integrin Signaling
- Section II: Late Events and Biological Functions of Integrin Signaling
- Section III: Inside-Out Signaling by Integrins
- Section IV: General Methods for Signaling Studies of Cell Adhesion Molecules
- Index
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Signaling Through Cell Adhesion Molecules by Jun-Lin Guan in PDF and/or ePUB format, as well as other popular books in Medicine & Biochemistry in Medicine. We have over one million books available in our catalogue for you to explore.