
eBook - ePub
Photosensitization of Porphyrins and Phthalocyanines
Ichiro Okura
This is a test
Share book
- 252 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Photosensitization of Porphyrins and Phthalocyanines
Ichiro Okura
Book details
Book preview
Table of contents
Citations
About This Book
Photosensitization of Porphyrins and Phthalocyanines covers the scentific background to porphyrins and phthalocyanines, and applications of the compounds, especially for the application for photosensitization. It also has a review of advances in research and applications in this field.
Frequently asked questions
How do I cancel my subscription?
Can/how do I download books?
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
What is the difference between the pricing plans?
Both plans give you full access to the library and all of Perlego’s features. The only differences are the price and subscription period: With the annual plan you’ll save around 30% compared to 12 months on the monthly plan.
What is Perlego?
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Do you support text-to-speech?
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Is Photosensitization of Porphyrins and Phthalocyanines an online PDF/ePUB?
Yes, you can access Photosensitization of Porphyrins and Phthalocyanines by Ichiro Okura in PDF and/or ePUB format, as well as other popular books in Medicine & Biochemistry in Medicine. We have over one million books available in our catalogue for you to explore.
Information
Part I
Synthesis and Characterization of Porphyrins and Phthalocyanines
Chapter 1 Preparation of Porphyrins and Phthalocyanines
1.1 General Methods for Preparation of Porphyrins
Porphyrins are classified into three categories: (A) porphyrins prepared from natural heme, (B) alkyl-substituted porphyrins and (C) meso-tetraphenylporphyrin and its derivatives. In this section, the preparation methods of the representative porphyrins in each category are described. The preparation methods of water-soluble porphyrins (D), metalloporphyrins (E) and chlorin derivatives (F) are also provided.
1.1.1 Synthesis of porphyrins from natural heme (Scheme 1-1)
Protoporphyrin IX (1) is prepared from protohemin as a starting material. Iron ion in the protohemin is removed by refluxing with iron in formic acid. After demetallation, protoporphyrin IX is obtained by the addition of ammonium acetate. Protoporphyrin IX methylester (2) is synthesized by esterification of protoporphyrin IX in HCl-methanol solution.1)
Hematoporphyrin IX (3) is prepared as follows. Protoporphyrin IX is treated with HBr-acetic acid to convert the vinyl group to a hydroxyl group and is then neutralized by NaOH. By the addition of excess NaOH solution, hematoporphyrin IX is obtained as a precipitate.
meso-Porphyrin IX (4) dimethylester is prepared as follows.2) The vinyl group of hematoporphyrin IX dimethylester is reduced to an ethyl group by hydrogen gas catalyzed by palladium-active carbon under methylmethacryrate and formic acid. meso-Porphyrin IX dimethylester is easily recrystallized from chloroform-methanol. This compound is most popular among the porphyrins from natural heme.
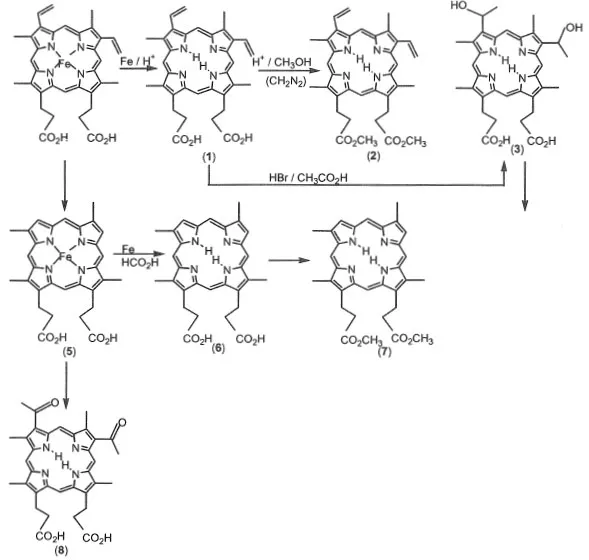
Scheme 1-1 Synthesis of porphyrins from natural heme.
Deuteroporphyrin IX (5) is prepared from protohemin as the starting material.3) Deuterohemin is produced by refluxing protohemin with resorcinol. Iron ion in deuterohemin is removed by refluxing with iron in formic acid. After demetallation, deuteroporphyrin IX methylester is obtained by esterification of protoporphyrin IX in HCl-methanol solution. 2,4-Diacetyldeuteroporphyrin IX is synthesized by the acylation of deuterohemin treated with acetic anhydride and tin chloride.3)
2,4-Diformyldeuteroporphyrin IX is prepared by the oxidation of protoporphyrin IX dimethylester using KMnO4/MgSO4 as oxidizing reagents in acetone. 2,4-Diformyldeuteroporphyrin IX is insoluble in organic solvents.4)
1.1.2 Synthesis of alkyl-substituted porphyrins
The representative alkyl-substituted porphyrins are etioporphyrin (EtioP) and octaethylporphyrin (OEP).5) There are three synthesis routes of OEP, as shown in Scheme 1-2.
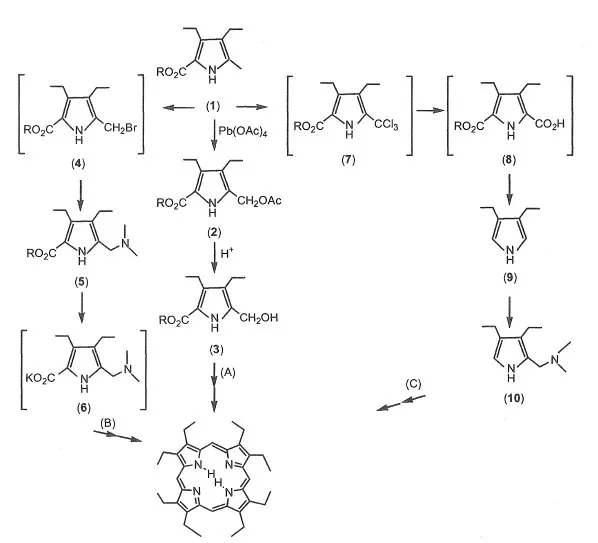
Scheme 1-2 Synthesis routes of OEP.
Route A5): The derivative (2) is synthesized by the acylation of a-methyl of pyrrole derivative (1) using Pb(OAc)4. The derivative (3) is prepared by hydrolysis of (2) in alkaline solution. OEP is synthesized by cyclization and oxidation of (3). The yield is about 20-30%. OEP is purified using alumina column chromatography and recrystallization from chloroform and methanol.
Route B6): The derivative (4) is synthesized by bromination of (1). The diethylamino-methyl derivative (5) is synthesized by nucleophilic substitution of amine to (4). The potassium salt (6) is obtained by hydrolysis of (5). OEP is synthesized by cyclization and oxidation of (6). The yield is about 20-30%. OEP is purified using alumina column chromatography and recrystal-lization from chloroform and methanol.
Route C7): 3,4-Diethylpyrrol (9) is prepared by decarboxylation of (8). The derivative (10) is synthesized by aminomethylation of (9) in formaldehyde and dimethylformamide. OEP is prepared by cyclization and oxidation of (10). The yield is about 20-30%. OEP is purified using alumina column chromatography and recrystallization from chloroform and methanol.
Octamethylporphyrin (OMP) is synthesized using the above methods.8) The yield of OMP is very low and OMP is insoluble in organic solvent.
1.1.3 Synthesis of meso-tetraphenylporphyrins
Rothemund synthesized meso-tetraphenylporphyrin(TPP) by condensation between pyrrole and benzaldehyde.9) Since this report, meso-tetraphenylporphyrin derivatives are synthesized using benzaldehyde derivatives. The yield of porphyrin improves especially when propionic acid is used as a solvent. Porphyrins are usually purified using column chromatography (silica gel or almina).10) The synthesis route is shown in Scheme 1-3. Tetranitrophenylporphyrin, tetratolylporphyrin, tetra(4-pyridyl) porphyrin and tetra(pentafluorophenyl)porphyrin are synthesized by this method.
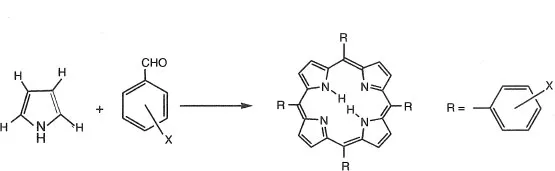
Scheme 1-3 Synthesis routes of meso-tetraphenylporphyrin derivatives.
1.1.4 Synthesis of water-soluble porphyrins
Water-soluble porphyrins, tetraphenylporphyrin tetrasulfonate (TPPS), is synthesized by the sulfonation of TPP using sulfuric acid. Tetrakis-(aminophenyl)porphyrin (TAPP) is synthesized by the reduction of tetrakis(4-nitrophenyl) porphyrin under Sn / HCl condition. Tetrakis-(4-carboxyphenyl)porphyrin (TCPP) is synthesized by hydrolysis of tetrakis-(4-methylcarboxyphenyl)-porphyrin in alkaline alcohol solution. Tetrakis-(4-methylpyridyl)porphyrin (TMPyP) is prepared by quaterization of tetra(4-pyridyl)porphyrin with methyliodide or p-toluenesulfonate methylester in DMF.
1.1.5 Synthesis of metalloporphyrins
Metalloporphyrins are widely use...