
eBook - ePub
Drug Toxicity and Metabolism in Pediatrics
Sam Kacew
This is a test
- 332 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Drug Toxicity and Metabolism in Pediatrics
Sam Kacew
Book details
Book preview
Table of contents
Citations
About This Book
The aim of this text is to examine the physiological development of the fetus. It allows the reader to study the unique pharmacokinetic and metabolic features of newborns and gives specific examples of drug metabolism in the newborn. The purpose of this book is to enhance the current knowledge of pharmacology of the newborn by observing the embryo and placenta in normal and abnormal development, placental transfer of drugs, metabolic pathways, and metabolism of specific drugs such as theophylline, benzodiazepines, and antibiotics. This is a useful book for those involved in pediatric research, pharmacology, toxicology, experimental therapeutics and biology.
Frequently asked questions
How do I cancel my subscription?
Can/how do I download books?
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
What is the difference between the pricing plans?
Both plans give you full access to the library and all of Perlego’s features. The only differences are the price and subscription period: With the annual plan you’ll save around 30% compared to 12 months on the monthly plan.
What is Perlego?
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Do you support text-to-speech?
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Is Drug Toxicity and Metabolism in Pediatrics an online PDF/ePUB?
Yes, you can access Drug Toxicity and Metabolism in Pediatrics by Sam Kacew in PDF and/or ePUB format, as well as other popular books in Medicina & Patologia. We have over one million books available in our catalogue for you to explore.
Chapter 1
Developmental Aspects of Pediatric Pharmacology and Toxicology
S. Kacew and S. Lock
TABLE OF CONTENTS
- I. Introduction
- II. Fetal Drug Exposure During Pregnancy
- III. Drugs and Breast Feeding
- IV. Drugs and the Child
- V. Drugs and the Older Child
- VI. Summary
- References
I. Introduction
The basic principles of pediatric drug therapy and toxicity were initially founded on the premise that the infant was simply a small adult. Over the last 20 years, it has been found that this concept is therapeutically inaccurate. Of necessity this has led to intensive investigation into the identification and elucidation of developmental parameters that influence clinical pharmacology. Within the field of pediatric pharmacology, it is generally accepted that there are a number of unique developmental stages which must be considered with respect to drug effect and toxicity. The developmental stages are related to the mode of exposure as follows:
- In utero the fetus may be inadvertently exposed to drugs secondary to maternal chemical ingestion.
- The neonate may encounter drugs as a result of maternal administration via the breast milk. At this stage, direct drug exposure can also occur.
- The older infant and child are no longer subject to lactational exposure, but merely to the direct route.
As the infant develops, the mother’s influence diminishes with respect to drug exposure. However, the direct route gains prominence with increasing age and can be potentially more hazardous. The aim of this chapter is to provide a general background on the influence of drugs on the developing infant.
II. Fetal Drug Exposure During Pregnancy
During the course of her pregnancy, a mother is likely to take a number of drugs for therapeutic reasons. In addition, with many more women in the work force, there is an increased potential for interactions between therapeutic agents and a variety of chemicals present in the occupational environment. Furthermore, a large number of women indulge in a variety of recreational chemicals. Alcohol, as an example, is well known to influence drug action. The consequences attributed to the exposure to a pharmaceutical product should be advantageous to the mother; however, in many instances, the effects can be deleterious to the fetus. Thus, it may be stated that the fetus is at some jeopardy as a result of exposure to pharmaceutical agents.
Nutrients essential for fetal growth and development require an active transport system to be moved from the maternal circulation to the fetal circulation against a concentration gradient. By contrast, drugs cross the placenta by simple diffusion. There are a number of factors listed in Table 1 which will influence the transfer of drugs from the maternal circulation to the fetus and thus will have a bearing on any subsequent fetal manifestations. The amount of a chemical that is transferred to the fetus is dependent on lipid solubility, the degree of ionization, and the molecular weight. Lipophilic drugs tend to diffuse across the placenta readily, while highly ionized compounds penetrate the placental membrane slowly. The molecular weight of an agent affects placental transfer, with the larger molecules crossing the placental barrier less readily. Protein binding of a drug or its metabolites will affect the rate and amount that is transferred to the fetus. Exposure of fetal target tissues to chemical entities may also be influenced by metabolism in the placenta or the fetal liver.2
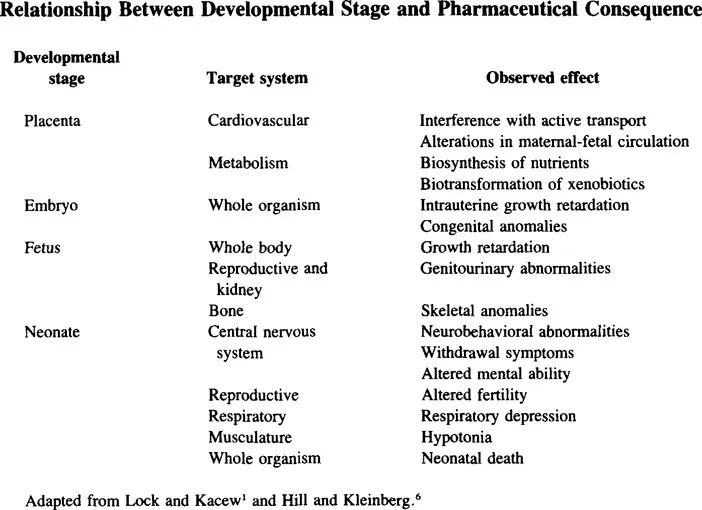
An important component to consider is the stage of fetal development at the time of chemical exposure. During the first week of development after fertilization, the embryo undergoes the process of cleavage and gastrulation. Exposure to drugs such as antimetabolites, ergot alkaloids, or diethylstilbestrol at this stage can result in termination of pregnancy.3 Organogenesis is the next developmental stage covering weeks two to eight of gestation. Exposure to drugs including thalidomide, alcohol, lithium, phenytoin, and isotretoin during this phase can result in serious structural abnormalities.3,5 Chemicals such as cigarette smoke, heavy metals, or carbon monoxide may affect development during the remaining gestational period, ranging from 9 weeks to 9 months. Predominant effects are alteration in the differentiation of the reproductive and central nervous systems.3 Consequently, altered brain function and growth retardation are some of the principle adverse effects due to exposure at this stage. The relationship between stage of development and type of effect is illustrated in Table 2.
TABLE 1
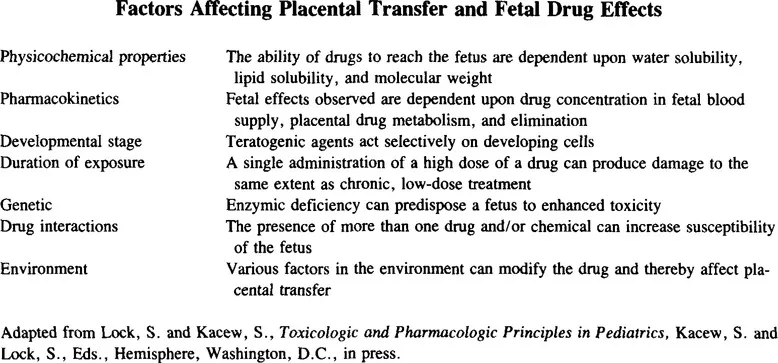
TABLE 2
A perspective of the potential seriousness of the problems associated with drug use during pregnancy and the consequence to the fetus is gained from examination of Tables 3 to 5. Although these tables are extensive, they are by no means all encompassing. Furthermore, some of the effects shown to occur in humans by one group of investigators could not necessarily be confirmed by other scientists. In spite of these uncertainties, any reports of potential adverse effects of a drug on the unborn child should be taken into consideration by a physician. Thus, there should be a clear indication that the benefits derived by the mother from a specific drug greatly outweigh the risk to the fetus. Tables 4 and 5 list selfadministered drugs (over-the-counter, OTC, preparations) and nontherapeutic drugs (drugs of abuse), respectively. There is, unfortunately, less than adequate awareness of the dangers associated with the use of OTC drugs.
TABLE 3


III. Drugs and Breast Feeding
The nursing mother can serve as a source of neonatal exposure to drugs. No matter whether the agent is an OTC medication or is prescribed by a physician, most drugs are detectable in breast milk. The presence of a drug in maternal milk may be construed as a potential hazard to the infant even though only 1 to 2% of total intake is likely to be found here.5 Hence, the primary consideration in maternal drug therapy is the risk to the nursing infant rather than the mere presence of a xenobiotic in the milk.
Several factors play a role in determining the quantity of a drug that will be transferred to breast milk. The amount of drug that is actually available for transfer to milk is dependent on certain maternal factors. As outlined in recent reviews,3,42 the dosage and frequency, as well as route of drug administration, are factors to consider in the mother. Following maternal intake, the pharmacokinetic principles of absorption, distribution, metabolism, and excretion of agent will play a role in determination of drug levels in the milk. The duration of exposure of the infant to lactational drugs can thus vary, depending upon maternal pharmacokinetics. If one considers the infant, the amount and frequency of feeding will affect the concentration of drug ingested. Further, the flow of blood to the breast, the composition of milk, and the rate of milk production, as well as resorption of drug from milk into the maternal circulation, are factors affecting the amount of drug reaching the infant. Finally, the most important factor influencing drug excretion into breast milk is the physicochemical features of the compound.3,42 The physicochemical characteristics, including degree of ionization, molecular weight, lipid solubility, and protein-binding capacity, will affect the ability to traverse the mammary gland epithelium and thus be available to the suckling infant. It is well established that drug use during lactation will in general result in the presence of these compounds in the milk. As indicated by Roberts,3 the critical factor to consider during breast feeding is whether the drug utilized poses a documented risk to the infant. From the information available, it is generally believed that few drugs fall into the category of agents which initiate significant and predictable toxicity. Certain drugs should b...