
- 288 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Chemistry of Plant Hormones
About this book
The chemistry of the five principal plant hormone groups is discussed in detail in this volume. Contributing authors review history and occurrence of each hormone group, methods of isolation and detection, biosynthesis and metabolism, and structural determination. Through these analyses, the authors clarify the role of endogenous plant growth regulators in the life cycle of higher plants. The text is supplemented with over 350 figures and structures of various plant hormones.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Chemistry of Plant Hormones by Nobutaka Takahashi in PDF and/or ePUB format, as well as other popular books in Biological Sciences & Biology. We have over one million books available in our catalogue for you to explore.
Information
Chapter 1
INTRODUCTION
The life cycle of higher plants is regular, though complex. Each stage, together with the shift to the next stage, is controlled by endogenous plant growth regulators. The situation is further complicated by the need for the life cycle to accomodate to environmental conditions such as light intensity, daylength, humidity, and nutritional conditions. Such responses to environmental change are believed to be due to the quantity and availability of endogenous plant hormones and other bioactive substances. In this sense the life cycle and its sensitive adjustment to outside stimuli may be due to the mediating role of such compounds, which thus fulfill a most important role.
At this stage we know of many kinds of plant growth regulators associated with a wide variety of physiological functions in higher plants. In many cases these have been isolated and chemically characterized. Among the most important of these are the so-called plant hormones (phytohormones).
The concept of plant hormones differs substantially from that of hormones in animals and insects, because the differentiation of organ tissues in plants is less extensive than in animals. Plant hormones can be broadly defined as follows: (1) they must be chemically characterized and shown to be biosynthesized in some plant organ, (2) they must be broadly distributed within the plant kingdom, (3) they must show specific biological activity in very low concentration and must be shown to play a fundamental role in regulating physiological phenomena in vivo, and (4) they are usually translocated within the plant from a biosynthesis site to an action site.
At present, five groups of plant growth regulators — auxins, gibberellins, cytokinins, abscisic acid, and ethylene — are regarded as plant hormones; however, the distinction between plant hormones and plant growth regulators other than plant hormones is not always easily seen. At this stage one new group of compounds that regulate plant growth, brassinolide and related compounds, must be considered as true plant hormones on account of their wide distribution in the plant kingdom and their unique biological activity. A less clear cut case is that of “florigen”, the hypothetical flower-inducing hormone. Logical as the hypothesis may be, the evidence to support the existence of such a hormone is incomplete and it has never been isolated. Despite this, it is logical to expect that various new plant hormones will be isolated and chemically characterized in the future; the wide variety of physiological function required to maintain the complicated life cycle of the higher plants requires that a considerable complexity of chemical control should exist.
Quite apart from these compounds that might be considered plant hormones, there are many natural products that show interesting physiological activity in the higher plants. In general these compounds are of limited distribution in the plant kingdom and show a rather restricted range of activities. They have been isolated not only from plant tissues but also as metabolites of microorganisms. They can be grouped according to their origin (plant, microorganism) and physiological activity as follows:
•Plant growth regulators of plant origin
1. Plant growth promotors (Figure 1)
Brassinolide and related compounds: brassinolide, castasterone, dolicholide, 6-deoxocastasterone, 2-deoxocastasterone, etc.
Strigol (germination stimulant from witchweed)
Phaseolic acid
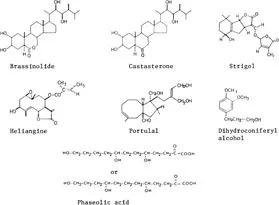
FIGURE 1. Structures of plant growth regulators of plant origin.
Dihydroconiferyl alcohol (synergist of gibberellin)
2. Plant growth inhibitors (Figure 2)
Benzoic acid and related compounds
Cinnamic acid and related compounds
Phenolic compounds including flavonoids
Unsaturated lactones: scoporetin, parasorbic acid, psoraen, seselin, etc.
Growth inhibitors isolated from Podocarpus: podolactones A—E, inumakilactones A— D, ponalactone A and its glucoside, hallactones A, B, sellowins A—C, nagilactones A—G, podolide, etc.
Momilactone and related compounds: momilactones A, B, annonalide Jasmonic acid and related compounds: jasmonic acid and its methyl ester, cucurbic acid and its glucoside
Asparagusic acid and related compounds: asparagusic acid and its sulfoxide, dihydroasparagusic acid, S-acetyldihydroasparagusic acid, etc.
Poly-yne-ene compounds: matricariaester, 2-(Z)-dehydromatricariaester, methyl 2-(Z)-decene-4,6-diynoate, etc.
Batatacins: batatacins I, II, III
Rooting inhibitors from Eucalyptus: G-regulators, G1, G2, G3, grandinol
Growth inhibitors in liverwort and algae: lunularic acid and related compounds
Growth inhibitors from bulbs of Lycoris radiata: lycoricidinol and lycoricidine
Others: harrintonolide, methyl pheoborides, juglone, 3-acetyl-6-methylbenzaldehyde, lignans with germination inhibitory activity, 4,8,13-duvatriene-1,3-diol
•Plant Growth Regulators of Microbial Origin
1. Plant growth promotors (Figure 3)
Helminthosporol and related compounds from Helminthosporium sativum: helminthosporoland cis-sativendiol, etc.
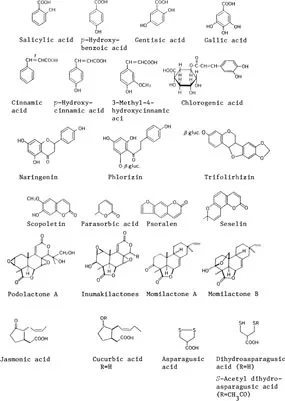
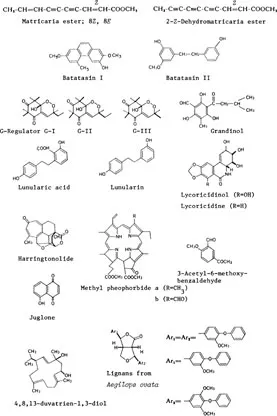
FIGURE 2. Structures of plant growth inhibitors.
Sclerin and related compounds from Sclerotinia spp.: sclerin, sclerotinins A, B
Malformins from Aspergillus spp. (malformation inducing substances): malformins A1, A2, B1, B2, C
Cotylenol and related compounds from Clodosporium sp.: cotylenol, cotylenins A—F
Radiclonic acid from penicillium sp.
Synergist to gibberellins from Pestalotia crytmeraecola and other unidentified fungi: pestalotin and related compounds
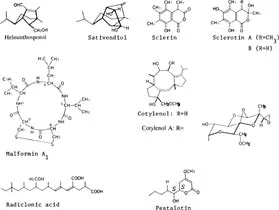
FIGURE 3. Structures of plant growth promotors of microbial origin.
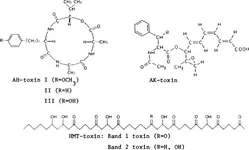
FIGURE 4. Structures of host-specific phytotoxins.
2. Plant growth inhibitors and phytotoxins
Host-specific toxins (Figure 4) have been isolated mainly from Helminthosporium and Alternaria spp. and have been shown to be used for host recognition by plant pathogens: AM-toxins I—III (Alternaria mali), AK-toxin (A. kikuchiana), HMT-toxins (Helminhosporium madys race T), HC-toxin (H. carbonum), toxin from A. alternata F. sp. cycopersici.
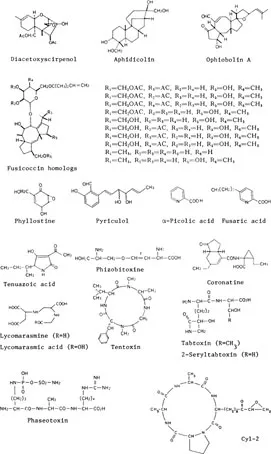
FIGURE 5. Structures of non-specific phytotoxins.
Nonspecific toxins (Figure 5) involve compounds with a wide variety of structures and cause very divergent symptoms on host plants. Some examples follow.
a. Terpenoids: diacetoxysciprenol (Fusarium equiseti), aphidicolin (Harziella entomophilla), ophiobolins (Ophiobolus and Helminthosporium spp.), Fusicoccins (Fusicoccum amygdali).
b. Other carbocyclic compounds: phyllosinol (epoxidon) and related compounds (Phyllosticta sp.).
c. Aromatic and heteroaromatic compounds: piryculol (Pyricularia oryzae), fusaric acid (Gibberella fujikuroi, Fusarium, and Nectria spp.), α-picolic acid (Pyricularia oryzae), tenuazoic acid (P. oryzae, Alternaria longipes).
d. Amino acids and peptides: rhizobitoxine and dihydro derivative (Rhizobium japonicum), coronatine (Pseudomonas coronafaciens), lycomarasmins (Aspergillus flavus and oryzae), tentoxin (Alternaria spp.), phaseotoxin (Pseudomonas phaseolicola), tabtoxin (Pseudomonas tabaci).
Clarification of the mechanism for the regulation of the life cycle in higher plants has been one of the most important areas of research in plant physiology. An ideal approach to such research should include all the following aspects: (1) isolation and characterization of endogenous compounds responsible for the physiological phenomenon; (2) exogenous application to other species to check for the appropriate phy...
Table of contents
- Cover
- Title Page
- Copyright Page
- Table of Contents
- Chapter 1 Introduction
- Chapter 2 Auxins
- Chapter 3 Gibberellins
- Chapter 4 Cytokinins
- Chapter 5 Abscisic Acid
- Chapter 6 Ethylene
- Index