
- 632 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
""Based on the plenary and invited lectures presented at the International Symposium on Micelles, Microemulsions, and Monolayers. Reviews the progress achieved in the last 25 years and describes new directions for research on micellar, microemulsion, and monolayer systems and their technological potential.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Micelles by DineshO. Shah in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Chemistry. We have over one million books available in our catalogue for you to explore.
Information
1
Micelles, Microemulsions, and Monolayers: Quarter Century Progress at the University of Florida
University of Florida, Gainesville, Florida
I. | MICELLES |
It is well recognized that a surfactant solution has three components: surfactant monomers in the aqueous solution, micellar aggregates, and monomers adsorbed as a film at the interface. The surfactant is in dynamic equilibrium among all these components. From various theoretical considerations, as well as experimental results, it can be said that micelles are dynamic structures whose stability is in the range of milliseconds to seconds. Thus, in an aqueous surfactant solution, micelles break and reform at a fairly rapid rate, in the range of milliseconds (1, 2, 3). Figure 1 shows the two characteristic relaxation times, τ1 and τ2, associated with micellar solutions. The shorter relaxation time, τ1, generally of the order of microseconds, relates to the exchange of surfactant monomers between the bulk solution and micelles, whereas the longer relaxation time, τ2, generally of the order of milliseconds to seconds, relates to the dissolution of a micelle after several molecular exchanges (4,5). It has been proposed that the lifetime of a micelle can be given by nτ2 where n is the aggregation number of a micelle (6). Thus, relaxation time τ2 is proportional to the lifetime of the micelle. A large value of τ2 represents a high stability of the micellar structure.
Figure 2 shows the relaxation time τ2 of micelles of sodium dodecyl sulfate (SDS) as a function of SDS concentration (4,7,8). It is evident that the maximum relaxation time of micelles is observed at 200 mM SDS concentration. This implies that SDS micelles are most stable at this concentration. For several years researchers at the Center for Surface Science and Engineering (CSSE) tried to correlate the relaxation time, τ2, with various equilibrium properties such as surface tension, surface viscosity, etc., but no correlation could be found. However, a strong correlation of τ2 with various dynamic processes such as foaming ability, wetting time of textile, bubble volume, emulsion droplet size, and solubilization of benzene in micellar solution was found (9).
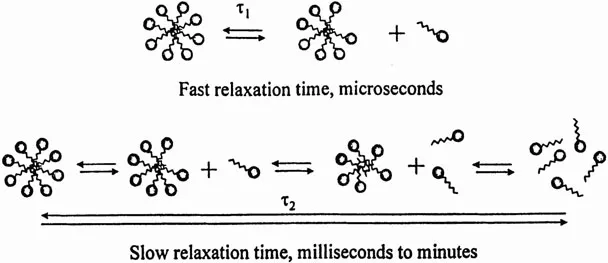
Figure 1 Two relaxation times of micelles, τ1, and τ2, and related molecular processes.
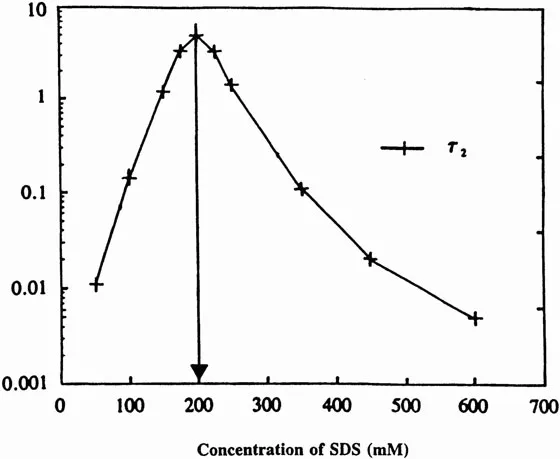
Figure 2 The relaxation time τ2 of SDS micelles as a function of SDS concentration.
Figure 3 schematically shows the effect of τ2 or micellar stability on the rate of adsorption of surfactant monomers at the air bubble surface. When a micellar solution has a large τ2 or high stability, it will provide less monomer to the newly created interface. Hence, the dynamic surface tension will be higher for micellar solutions having a large τ2. This will cause the volume of the bubble at the tip of the needle to be large. It is expected that a higher dynamic surface tension requires greater buoyancy force to break off the bubble from the tip of the needle. Thus, one expects a large bubble volume when the micelles are relatively stable, as represented by a large value of τ2 (10).
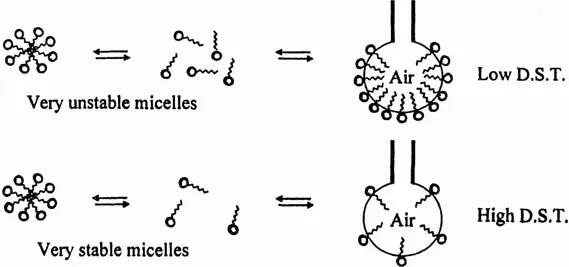
Figure 3 The effect of τ2 or micellar stability on the flux of monomers and on the rate of adsorption of surfactant at the air bubble/water surface. D.S.T., dynamic surface tension.
The foaming ability of a surfactant solution involves the sparging of air into a surfactant solution to create a large new interfacial area. The newly created surface consisting of foam lamellae has to be stabilized by surfactant molecules. When initial monomers present in a solution diffuse to the surface, micelles have to break down in order to provide the additional monomers. Thus, one expects that when micelles are relatively stable, less surfactant will diffuse to the interface and, hence, cause the film to break due to a lack of sufficient surfactant molecules at the interface (11). Figure 4 shows the effect of SDS concentration on τ2 and on foaming ability. It is evident that maximum τ2 corresponds to minimum foaming ability of SDS solution.
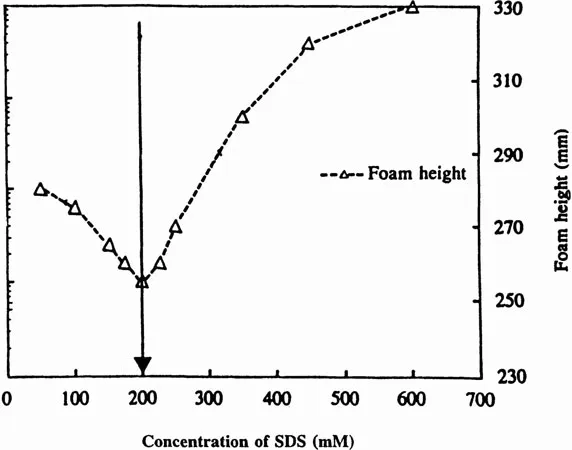
Figure 4 The effect of τ2 on foamability of SDS solutions at various concentrations.
Figure 5 schematically illustrates the effect of τ2 on wetting time of fabric. For fabric wetting to be effective, the surfactant monomers have to adsorb on the hydrophobic sites inherent on the fabric surface and convert them to hydrophilic sites. Therefore, the wetting effectiveness depends upon the amount of surfactant monomers available for adsorption. A relatively stable micelle provides less surfactant monomers and, hence, causes poor wetting and a longer wetting time. For experimental evaluation of this phenomenon, 1 in.2 pieces of various fabric were gently deposited on the surface of SDS solution. The amount of time before the fabric started sinking into the solution was measured. During this time, the solution penetrated into the fabric and replaced the air that had been trapped in the fabric (12). Figure 6 shows the effect of SDS concentration, and hence micellar relaxation time τ2, on the wetting time of cotton. It is evident that the maximum stability of micelles at 200 mM SDS concentration corresponds to the longest wetting time for cotton. Figure 7 shows the effect of SDS concentration, and hence micellar relaxation time τ2, on the wetting time of rayon. Similar studies were carried out with several different fabrics (13). It is evident that, in each fabric, the maximum wetting time occurs at 200 mM SDS concentration, although the magnitude of the maximum wetting time varies.
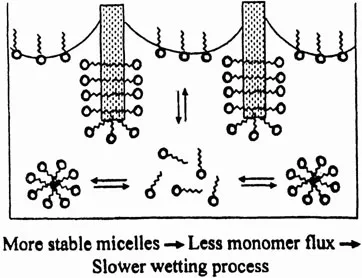
Figure 5 Schematic illustration of the effect of τ2 and hence micellar stability on wetting of fabric. Center for Surface Science and Engineering, University of Florida.
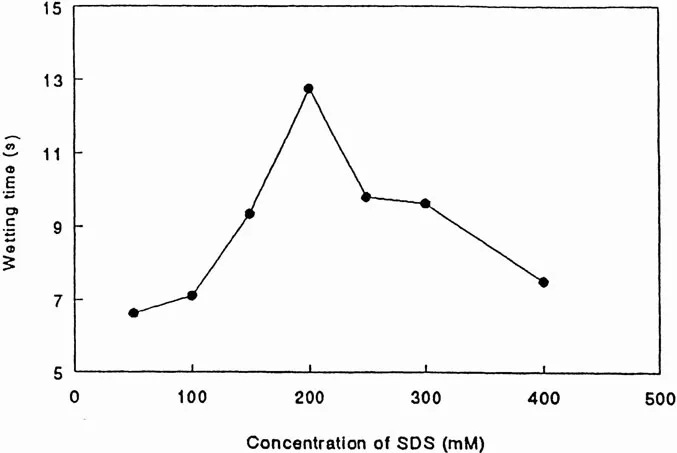
Figure 6 The effect of SDS concentrat...
Table of contents
- Cover
- Half Title
- Title Page
- Copyright Page
- Dedication
- Table of Contents
- Preface
- Contributors
- 1. Micelles, Microemulsions, and Monolayers: Quarter Century Progress at the University of Florida
- 2. Quarter Century Progress and New Horizons in Micelles
- 3. Recent Advances in Aqueous Surfactant Phase Science: Coexistence Relationships of the “Sponge” Phase
- 4. Surfactant Self-Assembly Structures at Interfaces, in Polymer Solutions, and in Bulk: Micellar Size and Connectivity
- 5. An Overview of Depletion and Surface-Induced Structural Forces in Thin Micellar Films
- 6. Structure and Design of Abnormally Long Thread-Like Micelles and Their Relation to Vesicles and Liquid Crystals
- 7. Quarter Century Progress and New Horizons in Microemulsions
- 8. New Developments in Polymerization in Bicontinuous Microemulsions
- 9. Application of Microemulsions in Soil Remediation
- 10. The Importance of Surfactant Hydrophobe Structure in Microemulsion Formation
- 11. The Role of Surfactants in Enhanced Oil Recovery
- 12. Nanosized Particles: Self-Assemblies, Control of Size and Shape
- 13. Microemulsions as Tunable Media for Diverse Applications
- 14. Double Emulsions Stabilized by Macromolecular Surfactants
- 15. Interparticle Forces from SANS Measurements of Frozen Dispersions
- 16. Phase Transitions in Lipid Monolayers at the Air-Water Interface
- 17. Surfactant Monolayers in Relation to Foam Breaking by Particles
- 18. Dynamic Adsorption and Tension of Spread or Adsorbed Monolayers at the Air-Water Interface
- 19. What X-rays Tell Us About Langmuir Monolayers
- 20. Inorganic Extended Solid Langmuir-Blodgett Films
- 21. Formation and Control of Unit Aggregates of Squaraines and Related Compounds in Langmuir-Blodgett Films
- 22. Protein and Molecular Assembly Monolayer and Multilayer Film Studies with Scanning Probe Microscopy
- 23. Self-Assembled Amphiphiles on Surface: “Surface Rheology”
- 24. Suprabiomolecular Architectures at Functionalized Surfaces
- 25. Langmuir-Blodgett Films of Condensation Polymers
- 26. Langmuir-Blodgett Film as Alignment Layers for Nematic Liquid Crystal Displays
- 27. Dye-Sensitized Solar Cells Based on Redox Active Monolayers Adsorbed on Nanocrystalline Oxide Semiconductor Films
- Index