
- 664 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Structure-Based Drug Design
About this book
Introducing the most recent advances in crystallography, nuclear magnetic resonance, molecular modeling techniques, and computational combinatorial chemistry, this unique, interdisciplinary reference explains the application of three-dimensional structural information in the design of pharmaceutical drugs. Furnishing authoritative analyses by world-renowned experts, Structure-Based Drug Design discusses protein structure-based design in optimizing HIV protease inhibitors and details the biochemical, genetic, and clinical data on HIV-1 reverse transcriptase presents recent results on the high-resolution three-dimensional structure of the catalytic core domain of HIV-1 integrase as a foundation for divergent combination therapy focuses on structure-based design strategies for uncovering receptor antagonists to treat inflammatory diseases demonstrates a systematic approach to the design of inhibitory compounds in cancer treatment reviews current knowledge on the Interleukin-1 (IL-1) system and progress in the development of IL-1 modulators describes the influence of structure-based methods in designing capsid-binding inhibitors for relief of the common cold and much more!
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
Topic
MedicineSubtopic
Pharmacology20
The Integration of Structure-Based Design and Directed Combinatorial Chemistry for New Pharmaceutical Discovery
3-Dimensional Pharmaceuticals, Inc., Exton, Pennsylvania
I. NEW CHALLENGES FOR DRUG DISCOVERY
Rapid advances in cell and molecular biology, together with comprehensive genome sequencing efforts, are providing detailed correlations between specific pathological conditions and discrete molecular targets. The same tools of recombinant DNA technology that identify key gene targets also provide the means for target biosynthesis in quantities sufficient for both the high-throughput screening of compound libraries for leads and the structure-based refinement of leads using x-ray crystallography and NMR spectroscopy.
The rapid expansion in genomics data makes it inevitable that targets will be identified whose functions are so poorly understood that the most rapid and efficient way to establish their involvement in disease will be through the development of prototype drugs. New approaches to drug discovery that are able to integrate many different types of information are needed to seize this opportunity and drive an optimally efficient discovery process.
In what follows, we describe a practical integration of structure-based design and combinatorial chemistry aimed at enhancing the effectiveness of both approaches. Three-dimensional structures provide the information required to most efficiently direct the design and optimization of new lead compounds. Combinatorial chemistry technologies, which are based on high-throughput automated methods of chemical synthesis, produce new classes of lead compounds and permit the rapid generation of structure-activity relationships (SAR). Chemi-informatics plays a key role in this integration by assuring that properties important in drug development are both factored into compound design and cumulatively tracked throughout the discovery process (Figure 1). The product of this approach is a permanently useful set of drug-design parameters. The integration of these technologies promises to produce an increase in the efficiency of drug discovery and may ultimately offer a means for reducing the aggregate failure rate of compounds selected for development. One paradigm for achieving the integration of these technologies is described below.
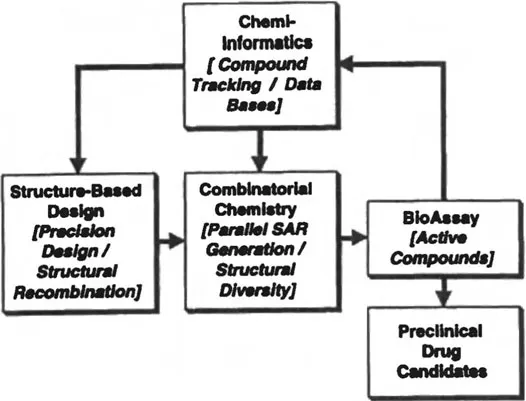
Figure 1 An integrated technology for drug discovery combines the precision of structure-based design with the parallelism of combinatorial synthesis. Chemi-informatics systems track and integrate all data emerging from the discovery cycle.
II. STRUCTURE-BASED DESIGN
Structure-based drug design has become a highly developed technology that is in active use in most major pharmaceutical companies. Structure-based design is an iterative process in which lead compounds identified by screening, de novo design, or mechanism-based features are systematically elaborated to improve potency and specificity [1,2]. The process involves successive rounds of structure determination of lead-target complexes, design of lead modifications using molecular modeling tools, synthesis of new drug leads, and measurement of the chemical and biological properties of the modified leads using screens for target function. Iterative refinement and optimization of drug leads is an effective strategy for generating potent preclinical candidates. Structure-based design can also be used to design new chemical classes of compounds that present similar substituents to the target using a template or scaffold which is chemically distinct from previously characterized leads [3,4].
Structure determination typically relies on x-ray crystallography or highfield nuclear magnetic resonance to directly visualize the 3-dimensional structure of a molecular target and the structures of complexes of the target with drug leads. Alternately, many targets fall into identifiable classes that frequently enable the development of homology models of the 3-dimensional target structure or a mechanism-based strategy for drug-lead generation. Ongoing genome sequencing efforts have led to the identification of hundreds of potential therapeutic targets, many of which represent possible sources of crossover pharmacology. Homology modeling is a key feature of an integrated drug discovery effort because it allows this genomics information to be utilized early in the development of target ligands or in the engineering of ligand specificity.
Although structure-based design is an effective technology, current limitations center on the inability to quantitatively predict how specific modifications of the lead will actually affect ligand binding affinity [5,6]. This reflects the complexity of the drug-binding process and our inability to accurately predict the conformational response of macromolecular structures to ligand binding. In addition, we have only limited ability to accurately calculate molecular energy parameters or to accurately estimate the effects of factors such as polarizability, solvation, and entropy that may have an important influence on drug-binding energetics. Although computational methods will continue to improve, most design work (and algorithms) still relies heavily on heuristic rules (Figure 2) that have been developed through experience and that guide the structural and medicinal chemists in the systematic modification of lead compounds [7]. As a practical consequence, many cycles of serial lead modification are required in order to produce molecules of suitable potency and specificity to be considered preclinical drug candidates.
Structural information can increase the efficiency with which pharmacokinetic or toxicological liabilities in lead compounds are eliminated by suggesting where compounds can be modified so as to alter drug properties without affecting target potency. Structural data can also be used to direct de novo design of alternate and distinct chemical classes of lead compounds, each of which might be expected to have a different pharmacological profile [3,4]. New chemical compound classes can also be designed from existing lead compounds by recombining substituents and core regions (scaffolds) from existing lead compounds. Chemically distinct lead series can then be optimized in parallel so that when a preclinical candidate is found to have inadequate drug properties, a backup is immediately available for preclinical evaluation. As outlined below, both the scaffold modification and structural recombination strategies are key components of an integrated drug discovery technology that combines structure-based design and combinatorial chemistry.
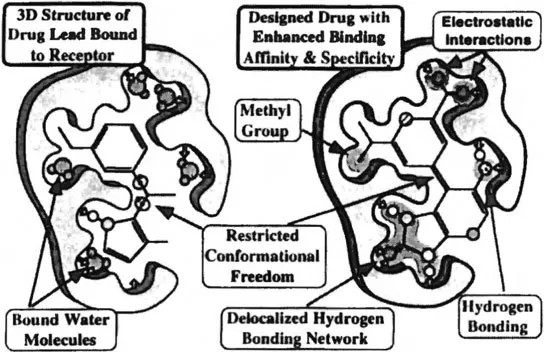
Figure 2 Some beuriitic rules frequently used in structure-based drug design. The positions of “bound” water molecules are key indicators for lead modification sites.
III. COMBINATORIAL CHEMISTRY
Combinatorial chemical technology enables the parallel synthesis of organic compounds through the systematic addition of defined chemical components using highly reliable chemical reactions and robotic instrumentation [8, 9, 10, 11], Large libraries of compounds result from the combination of all possible reactions ...
Table of contents
- Cover
- Half Title
- Title Page
- Copyright Page
- Table of Contents
- Preface
- Contributors
- AIDS
- ARTHRITIS AND INFLAMMATION
- CANCER
- DIABETES MELLITUS
- HEART DISEASES
- PARKINSON’S DISEASE
- SLEEPING SICKNESS
- IMMUNE DISEASES AND CYTOKINES
- ANTIVIRALS
- NOVEL METHODOLOGIES
- Index
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Structure-Based Drug Design by Pandi Veerapandian in PDF and/or ePUB format, as well as other popular books in Medicine & Pharmacology. We have over one million books available in our catalogue for you to explore.