
eBook - ePub
Physical Chemistry for Chemists and Chemical Engineers
Multidisciplinary Research Perspectives
- 360 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Physical Chemistry for Chemists and Chemical Engineers
Multidisciplinary Research Perspectives
About this book
This volume is based on different aspects of chemical technology that are associated with research and the development of theories for chemical engineers, helping to bridge the gap between classical analysis and modern, real-life applications. Taking an interdisciplinary approach, the authors present the current state-of-the-art technology in key materials with an emphasis on the rapidly growing technologies.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Physical Chemistry for Chemists and Chemical Engineers by Alexander V. Vakhrushev, Reza Haghi, J.V. de Julián-Ortiz, Alexander V. Vakhrushev,Reza Haghi,J.V. de Julián-Ortiz in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Biotechnology. We have over one million books available in our catalogue for you to explore.
Information
PART I
Bioscience and Technology
CHAPTER 1
UNSATURATED BIODEGRADABLE POLY (ESTER AMIDE) COMPOSED OF FUMARIC ACID, L-LEUCINE, AND 1,6-HEXANEDIOL
1Faculty of Chemical Technology and Metallurgy, Department of Chemical and Biological Engineering, Georgian Technical University, 77, Kostava Str., 0175 Tbilisi, Georgia
2Ivane Beritashvili Center of Experimental Biomedicine, 14, Gotua St., 0160 Tbilisi, Georgia
*Corresponding author. E-mail: [email protected]
CONTENTS
Abstract
1.1 Introduction
1.2 Experimental Part
1.3 Discussion of Results
Keywords
References
ABSTRACT
Unsaturated poly (ester amide) (PEA) containing unsaturated double bonds in the backbones has been synthesized by the interaction of di-p-nitrophenyl fumarate with di-p-toluenesulfonic acid salt of bis-(L-leucine)-1,6-hexylene diester in solution under conditions of active polycondensation. Optimal reaction conditions of polymer synthesis have been studied and unsaturated L-leucine-based PEA soluble in organic solvents has been obtained for the first time.
1.1 INTRODUCTION
Poly (ester amide) (PEA) is a relatively new family of biodegraded polymers on the basis of natural amino acids, aliphatic diols, and dicarboxylic acids. The complex of positive properties inherent for aliphatic polyesters and polyamides is combined in these polymers, namely: biodegradation skills (polyesters), hydrophilic property, high biocompatibility with tissues, and desirable mechanical properties in case of average (30,000–50,000) molecular masses (polyamides)1,2 PEAs are prospective materials from the viewpoint of their use in surgery, pharmacology, and tissue engineering. Further improvement of PEA properties and respectively, extension of the sphere of their application is possible through their functionalization and insertion of chemically active groups3 or hydrophobic groups4 into polymeric chains. Functionalization of polymers gives an opportunity to bind medications and bioactive substances with them using chemical bonds and to carry out their multiple transformations aimed to further upgrading of their properties, and so forth.
One of the prospective ways of functionalization of polymers is the insertion of unsaturated bonds both into the main chain and lateral chains of macromolecules.5,6 Through adjoining to unsaturated bonds is possible to carry out insertion of desirable functional groups into macromolecules, as well as multiple grafting reactions, structurization (cross-linking) of polymers, copolymerization, hybridization with other unsaturated polymers, for example, with unsaturated polysaccharides (acryloyl dextran, etc.) for receipt of multifunctional biodegraded hydrogels, and so forth.
Recently, we have synthesized unsaturated PEA (UPEA) on the basis of fumaric acid, L-phenylalanine, and 1,6-hexanediol.7 Obtained polymer after separation from reaction solution was dissolved only in m-cresol and trifluoroisopropanol and was not dissolved in solvents of saturated PEAs (dimethylformamide, chloroform)1 that can be ascribed to increased chain rigidity, which is caused by existence of double bonds in it and strong intermolecular hydrophobic interaction between benzyl groups of phenylalanine. At the same time, we have established earlier1 that PEAs received on the basis of amino acid L-leucine were better dissolved in organic solvents on the basis of L-phenylalanine compared with obtained analogs. That is why with the purpose of increase in solubility, we have decided to replace L-phenylalanine with L-leucine and to receive UPEA on the basis of corresponding derivatives.
1.2 EXPERIMENTAL PART
Synthesis of L-leucine-containing UPEA included three stages: (1) synthesis of basic unsaturated monomer, di-p-nitrophenyl fumarate (I); (2) synthesis of di-p-toluenesulfonate of bis-(L-leucine)-1,6-hexylene diester (II); and (3) polycondensation of (I) and (II) monomers.
The unsaturated active diester, di-p-nitrophenyl fumarate, and biselectrophilic monomer (I) were synthesized from fumaryl chloride and p-nitrophenol as starting materials.
In the present study, we have applied the interfacial synthesis method: into 1 l three-neck flask equipped with mechanical stirrer, 16.68 g (0.12 mol) of p-nitrophenol and 12.72 g (0.12 mol) of Na2CO3 were dissolved in 300 ml of water at the room temperature. The prepared solution was vigorously stirred and then 6.5 ml (0.06 mol) of fumaryl chloride in 100 ml of chloroform was added. Stirring was continued for additional 15 min until a solid precipitate was formed, which then was filtered off and washed thoroughly with water and dried in vacuum at 60°C. The crude product (I) was obtained in 86% yield with melting point (m.p.; 232–234°C. Single recrystallization from acetone was sufficient to obtain monomer with “polycondensation grade” purity (m.p. 235–237°C).
We have synthesized bis-nucleophilic monomer, di-p-toluenesulfonate of bis-(L-leucine)-1,6-hexylene diester (II), and purified them using previously described method (see ref 7 and 1, respectively).
1.3 DISCUSSION OF RESULTS
At the first stage of the study, the synthesis of UPEA was implemented according to Scheme 1.1.
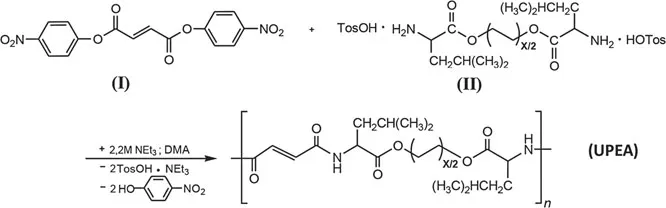
SCHEME 1.1 Polycondensation reaction of di-p-nitrophenyl fumarate (I) and di-p-toluenesulfonate of bis-(L-leucine)-1,6-hexylene diester (II) monomers.
Conditions for the synthesis of a saturated PEA under specified optimum conditions of polycondensation: solvent—dimethylacetamide, acid acceptor—triethylamine, monomers concentration—1.2 mol/l, and reaction temperature—80°C. After completion of reaction, in all cases, regardless of the fact whether the soluble polymer is formed or not, reaction mass was moved to water and precipitated polymer was thoroughly washed with water, dried, and finally washed by ethyl acetate at room temperature up to negative test on p-nitrophenol (ethyl acetate should not be discolored to yellow when adding the alkali).
At first, the polycondensation proceeded homogeneously, but after 10-12 min since the beginning of reaction, the reaction solution was heavily thickened and the gel was formed. The obtained polymer was not dissolved in organic solvents, including 1,1,2,2-tetrachloroethane/phenol (3:1), m-cresol, and hexafluoroisopropanol, in which the similar phenylalanine-containing polymers are soluble (as well as not crosslinked UPEAs, see below). Addition of 5% of LiCl to reaction area (which as a rule substantially increases the solubility of amide bond-containing polymers in amid solvents) has no desired effect (see Table 1.1). All this points at formation of cross-linked polymers that could be caused by intermolecular interaction of terminal amino groups of macromolecule with strongly electrophilic double bonds of residues of fumaric acid in polymer chain (that, in our opinion, takes place due to more flexibility of macromolecules of leucine polymer, compared with phenylalanine polymer) (Scheme 1.2).
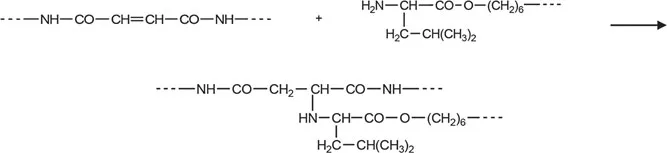
SCHEME 1.2 Intermo...
Table of contents
- Cover
- Half Title
- Title Page
- Copyright Page
- Table of Contents
- List of Contributors
- List of Abbreviations
- List of Symbols
- Preface
- PART I: Bioscience and Technology
- PART II: Nanoscience and Technology
- PART III: New Developments and Methods
- Index