
- 376 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Silicone Surfactants
About this book
The book offers a good summary of the field for all scientists who are interested in synthesis, properties, and the application of silicone surfactants." ---Molecular Chemistry and Physics. "Serves as a comprehensive introduction to the preparation, uses, and physical chemistry of silicone surfactants--focusing on silicone polyoxyalkylene copolymers that are surface active in both aqueous and nonaqueous systems. Covers applications in the manufacture of polyurethane foam, coatings, wetting agents, fabric finishes, and polymer surface modifiers."
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
1
Siloxane Surfactants
RANDAL M. HILL
Central Research and Development, Dow Corning Corporation, Midland, Michigan
- I. Introduction
- II. Molecular Structures and Nomenclature
- III. Synthesis and Chemistry
- A. Preparation of the siloxane backbone
- B. Transetherification
- C. Hydrosilylation
- D. Two-step synthesis using reactive intermediate
- E. Organophilic siloxanes and prepolymers
- F. Carbosilane surfactants
- G. Hydrolytic stability
- IV. Surface Activity
- A. Nonaqueous systems
- B. Aqueous systems
- C. Interfacial tension lowering
- D. Wetting and spreading
- E. Mixtures of siloxane and hydrocarbon surfactants
- F. Stabilization of colloidal dispersions
- V. Aqueous Aggregation Behavior
- A. Nonionic polyoxyethylene siloxane surfactants
- B. EO/PO-based siloxane surfactants
- C. Ionic siloxane surfactants
- VI. Ternary Phase Behavior
- VII. Applications
- A. Polyurethane foam manufacture
- B. Textile and fiber industry
- C. Personal care and cosmetic applications
- D. Paints and coatings
- VIII. Summary
- References
I. Introduction
Siloxane surfactants consist of a permethylated siloxane group coupled to one or more polar groups. This class of surfactants finds a variety of uses in applications where other types of surfactants are relatively ineffective [1, 2 and 3]. Siloxane surfactants have certain unique properties:
- Their hydrophobic group is silicone, so that
- They are able to lower surface tension to ≈ 20 dyn/cm compared with ≈ 30 dyn/cm for typical hydrocarbon surfactants, causing them to be
- Surface active in both aqueous and nonaqueous media.
In addition,
4. They are prepared by different chemistries, yielding molecular structures of different types and ranges [4,5], which are often fluid to very high molecular weights [6].
Siloxane surfactants were introduced to the marketplace in the 1950s for the manufacture of polyurethane foam [7]. Soon afterward other applications were invented for them [8]. Nonaqueous surface activity is the basis for their use in polyurethane foam manufacture, as demulsifiers in oil production, and as defoamers in fuels. Their ability to lower surface tension leads to wetting and spreading applications. Different molecular structures and high molecular weights make them useful as novel emulsifiers. Silicones impart a unique dry-lubricity feel to surfaces such as textiles, hair, and skin. Since siloxane surfactants incorporate silicone in a water-soluble or water-dispersible form, they represent a convenient means for putting silicone on a surface by way of an aqueous formulation.
The molecular origin of the principal difference between hydrocarbon and siloxane surfactants is illustrated in Fig 1. The surface active character of siloxane surfactants is due to the methyl groups, the—O—Si—O—Si—backbone simply serves as a flexible framework on which to attach the methyl groups [9, 10, 11 and 12]. The surface energy of a methyl-saturated surface is about 20 dyn/cm [9], and this is also the lowest surface tension achievable using siloxane surfactants. In contrast, most hydrocarbon surfactants consist of alkyl, or alkylaryl hydrophobes, which contain mostly —CH2— groups, and pack loosely at the air-liquid interface. The surface energy of such a surface is dominated by the methylene groups, and for this reason hydrocarbon surfactants typically achieve surface tensions of about 30 dyn/cm or higher [9]. Thus, the lower surface tensions given by siloxane surfactants can be traced directly to molecular structure, the unusual flexibility of the siloxane backbone, and the different surface energies of —CH3 versus —CH2—.
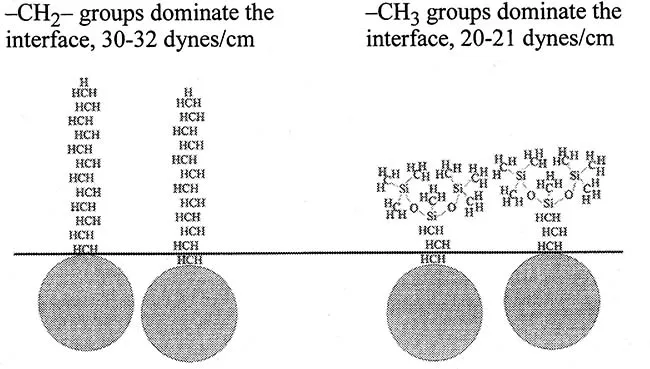
FIG. 1 Comparison of the surface character of hydrocarbon versus siloxane surfactants.
Siloxane surfactants are similar to hydrocarbon surfactants in many common features of surfactancy [2,13, 14 and 15]:
- There is a break in their surface tension versus log concentration curve reflecting the onset of self-association (such as micelle formation).
- Critical aggregation concentrations (cac) vary with molecular structure in the same way—within a homologous series, proportionately larger hydrophobic groups lead to smaller cac values.
- They show similar patterns of self-association in aqueous solution, forming aggregates and liquid crystal phases, of the same types and following the same trends with molecular structure.
- Siloxane surfactants incorporating polyoxyalkylene groups also show inverse temperature solubility and cloud points.
This last point requires some clarification: in the dilute concentration range, many siloxane surfactants form cloudy lamellar phase dispersions that are unrelated to the existence of a cloud point as it is usually understood [13].
Substantial advances in our understanding of this class of surfactants in recent years have covered their aqueous aggregation behavior, their ternary phase behavior with silicone oils, and their ability to promote rapid wetting of hydrophobic substrates. This chapter attempts to describe the structure, preparation, and surfactancy properties of this fascinating class of surfactants incorporating these recent advances. A brief discussion of some common applications also is given to illustrate how the unusual properties of siloxane surfactants are used. Detailed treatments of synthesis, superwetting, aqueous aggregation, and ternary phase behavior, and selected application topics are given elsewhere in this volume.
II. Molecular Structures and Nomenclature
Polydimethylsiloxane (PDMS) is itself surface active [16]. Gruning and Koerner [4] suggest a broad definition of siloxane surfactants to include all surface active copolymers containing a siloxane entity. We prefer to limit the scope to molecules with well-defined and well-separated hydrophilic and hydrophobic parts. Copolymers and terpolymers based on the PDMS backbone can be used to modify interfacial properties (one such use is illustrated in Fig. 2) and could be included under the umbrella of siloxane surfactants. There is a significant literature on such materials [17, 18, 19, 20, 21, 22 and 23], and they are discussed by Yilgor in this volume [24]. This introduction focuses primarily on siloxane surfactants that are useful in aqueous systems. Most siloxane surfactants are copolymers of PDMS and polyalkylene oxides of intermediate molecular weight. Nonaqueous surface ...
Table of contents
- Cover
- Half Title
- Title Page
- Copyright Page
- Preface
- Contents
- Contributors
- 1. Siloxane Surfactants
- 2. Silicone Polyether Copolymers: Synthetic Methods and Chemical Compositions
- 3. Novel Siloxane Surfactant Structures
- 4. Surface Activity and Aggregation Behavior of Siloxane Surfactants
- 5. The Science of Silicone Surfactant Application in the Formation of Polyurethane Foam
- 6. Silicone Polymers for Foam Control and Demulsification
- 7. Silicone Surfactants: Applications in the Personal Care Industry
- 8. Silicone Surfactants: Emulsification
- 9. Use of Organosilicone Surfactants as Agrichemical Adjuvants
- 10. Polymer Surface Modifiers
- 11. Surfactant-Enhanced Spreading
- 12. Ternary Phase Behavior of Mixtures of Siloxane Surfactants, Silicone Oils, and Water
- Index
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Silicone Surfactants by Randall M. Hill in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Chemistry. We have over one million books available in our catalogue for you to explore.