
Molecular Dynamics Simulation of Nanostructured Materials
An Understanding of Mechanical Behavior
- 314 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Molecular Dynamics Simulation of Nanostructured Materials
An Understanding of Mechanical Behavior
About this book
Molecular dynamics simulation is a significant technique to gain insight into the mechanical behavior of nanostructured (NS) materials and associated underlying deformation mechanisms at the atomic scale. The purpose of this book is to detect and correlate critically current achievements and properly assess the state of the art in the mechanical behavior study of NS material in the perspective of the atomic scale simulation of the deformation process. More precisely, the book aims to provide representative examples of mechanical behavior studies carried out using molecular dynamics simulations, which provide contributory research findings toward progress in the field of NS material technology.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
1 | Structural Description of Materials |
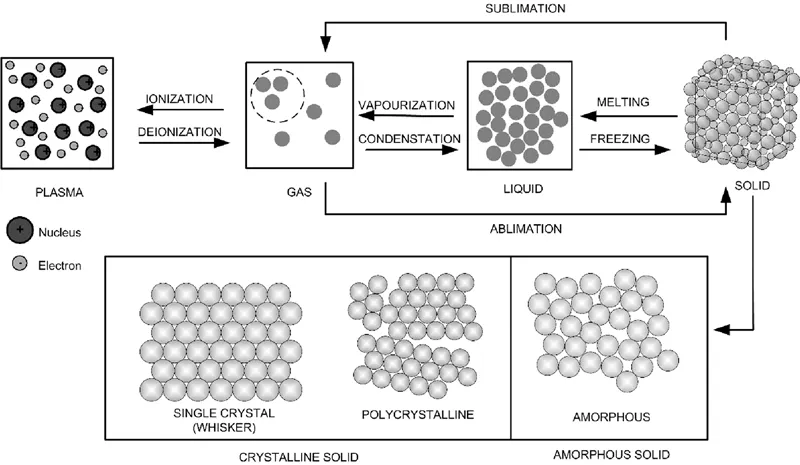
1.1 ATOMIC ARRANGEMENTS IN MATERIALS
1.1.1 PERIODICITY IN CRYSTALS AND SYMMETRY ELEMENTS
Type of Solid | Form of Unit Particles | Forces Between Particles | Solubility | Examples |
|---|---|---|---|---|
Molecular | Atoms or molecules | London dispersion forces, dipole-dipole forces, hydrogen bonds | Depends on the polarity of the molecules | Argon (Ar), methane (CH4), sucrose (C12H22O11), dry ice (CO2) |
Covalent network | Atoms connected in a network of covalent bonds | Covalent bonds | Insoluble in all solvents | Diamond (C) and quartz (SiO2) |
Ionic | Positive and negative ions | Electrostatic attractions | Soluble in polar solvents | Typical salts such as NaCl and Ca(NO3)2 |
Metallic | Atoms | Metallic bonds | Insoluble in all solvents | All metallic elements: Cu, Fe, Al, Pt, etc |
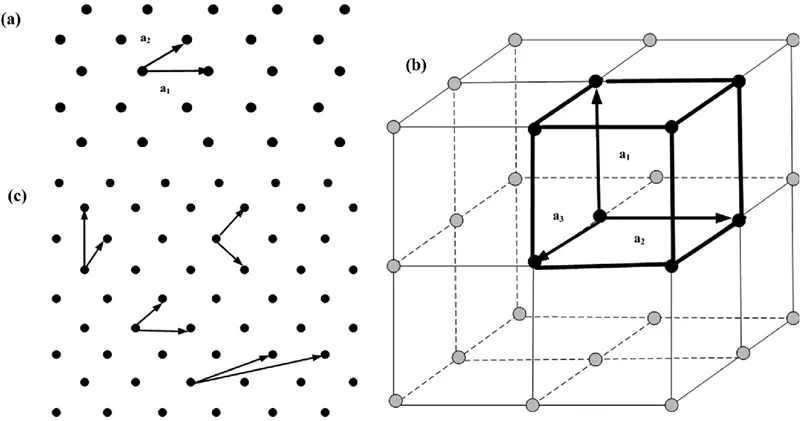
1.1.2 CRYSTAL LATTICES AND STRUCTURES
Sl. No. | Crystal System | Relation Between the Sides | Relation Between the Angles | Bravais Lattices, Number and Types |
|---|---|---|---|---|
1. | Cubic | a = b = c | α = β = γ = 90° | 3 = S, BC, FC |
2. | Tetragonal | a = b ≠ c | α = β = γ = 90° | 2 = S, BC |
3. | Hexagonal | a = b ≠ c | α = β = 90°, γ = 120° | 1 = S |
4. | Orthorhombic | a ≠ b ≠ c | α = β = γ = 90° | 4 = S, BC, FC, EC |
5. | Rhombohedral | a = b = c | α = β = γ ≠ 90° | 1 = S |
6. | Monoclinic | a ≠ b ≠ c | α = β = 90°, γ ≠ 120° | 2 = S, EC |
7. | Triclinic | a ≠ b ≠ c | α ≠ β ≠ γ ≠ 90° | 1 = S |
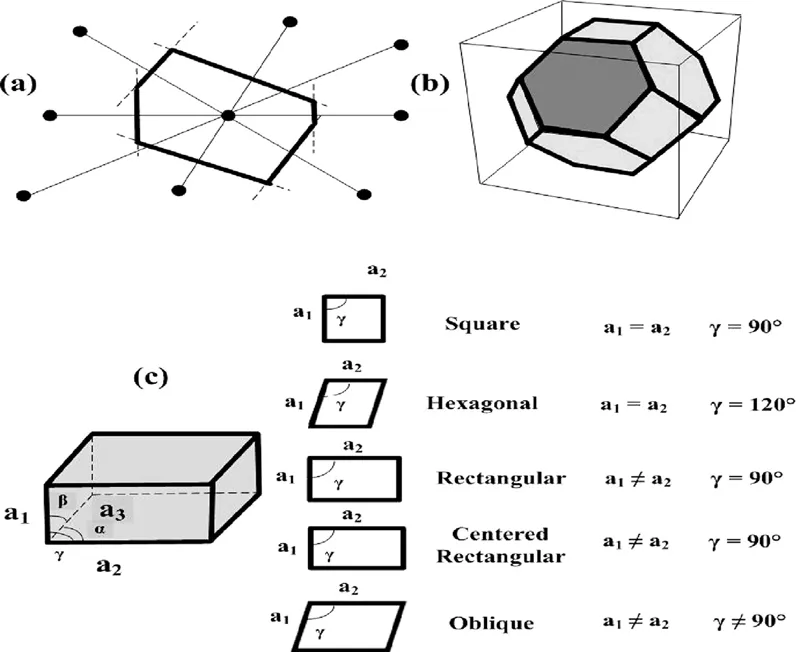
Table of contents
- Cover
- Half Title
- Title Page
- Copyright Page
- Dedication
- Table of Contents
- Preface
- Authors
- Chapter 1 Structural Description of Materials
- Chapter 2 Mechanical Behavior of Materials
- Chapter 3 Creep and Fatigue Behavior of Materials
- Chapter 4 Mechanical Behavior of Nanostructured Materials
- Chapter 5 Basics of Molecular Dynamics Simulation
- Chapter 6 Stress-Strain Behavior Investigation by Molecular Dynamic (MD) Simulation
- Chapter 7 Fracture Simulations Using Molecular Dynamics (MD)
- Chapter 8 Creep Behavior Investigation by Molecular Dynamics (MD) Simulation
- Chapter 9 Fatigue Behavior Investigation by Molecular Dynamics (MD) Simulation
- Index
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app