
- 368 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Surface Analysis of Paper
About this book
First published in 1995, Surface Analysis of Paper examines surface analysis techniques from a paper industry perspective and places heavy emphasis on applications. Modern techniques, including ion mass spectrometry, infrared spectroscopy, and optical profilometry are reviewed in a straightforward manner. This new book provides details on widely used methods and instruments, and discusses how they can be used to attain, for example, contour maps of the microscopic constituents on paper surfaces and accurate analyses of the physical properties of paper.
Organized into three sections, Surface Analysis of Paper provides thorough coverage of the physical characteristics of paper, and a clear picture of new and emerging analytical methods. Carefully chosen background material on fundamental concepts is included wherever such material assists in understanding the uses of analysis methods.
Each chapter contains:
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
6
FT-IR Spectroscopy
* Institute of Paper Science and Technology, Atlanta, Georgia, U.S.A.
INTRODUCTION
SAMPLING TECHNIQUES
NEAT FILM
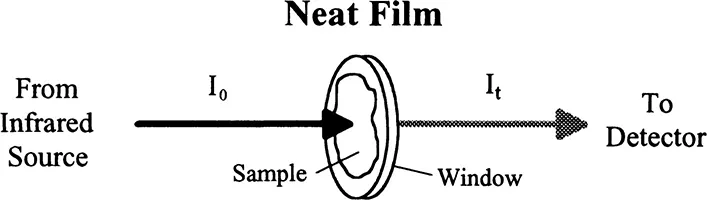
Table of contents
- Cover
- Half Title
- Title Page
- Copyright Page
- Table of Contents
- I. Physical Characterization of Surfaces
- II. Spectroscopic Methods
- III. Emerging Technologies
- Index
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app