![]()
Section II
Advanced Glycation Endproducts
![]()
6
Advanced Glycation Endproducts
An Introduction
Andreas Simm* and Alexander Navarrete Santosa
Martin-Luther-University Halle-Wittenberg, Department of Cardiac Surgery, Ernst-Grube Str. 40, Halle (Saale), Saxony-Anhalt Germany, D-06120.
Advanced Glycation Endproducts (AGEs) are the result of the Maillard reaction. AGEs arise from the non-enzymatic reaction of reactive carbohydrates (sugars) with DNA, proteins or lipids. There is some confusion in the terminology of sugar modified proteins. This was nicely summarized by Rabbini and Thornalley 2012 (Rabbani and Thornalley 2012): In 1985, the Nomenclature Committee of International Union of Biochemistry and the International Union of Pure and Applied Chemistry recommended the term glycation for all reactions that link a sugar to a protein or a peptide, whether or not catalyzed by an enzyme. In 1993, a discrimination between enzymatic modification (glycosylation) and non-enzymatic modification (glycation) of proteins with sugars was proposed (Lis and Sharon 1993). Thereafter, the mix-up of the nomenclature still persisted, so for a long time many names existed for non-enzymatic modified proteins, such as glycated, glycosylated, or non-enzymatic glycosylated and so on. Nowadays, “glycated protein” is the accepted terminology for the products of the non-enzymatic reaction of proteins with sugars.
Since the detection of fire, food was heated, leading to browning and making it easily digestible nutrition with better taste and flavor. While the effect of heating on taste and color was known, the chemistry behind it was unclear for a long time. It was the French chemist Louis-Camille Maillard, who first described in 1912, a reaction between glucose and glycine during a heating process (Maillard 1912). This non-enzymatic browning reaction (using temperatures around 150ºC) was later named as the Maillard reaction. But it was only more than 40 years later, in 1953, that the chemist, John Edward Hodge, published the mechanism of the Maillard reaction (Hodge 1953). During the 1920s, the Italian chemist, Mario Amadori, concentrated on the condensation reaction between carbohydrates like glucose and amines like p-anisidine. This rearrangement after building of the first reaction product within the Maillard reaction, the Schiff-base, leading to a more stable product is now known as the Amadori rearrangement. The following reactions are more complex ending in the so called AGEs. Beside the “classical reaction” of a sugar with the amino group of amino acids, it is now well known that small oxidative degradation products of glucose, reactive α-oxoaldehydes like GO and MGO, are important inducers of AGEs. As early as 1913, the enzymatic system which converts methylglyoxal to lactate (the glyoxalase system) was discovered as methylglyoxalase (shortly glyoxalase) (Neuberg 1913). MGO and the regulating glyoxalase system was thought to regulate cell division and the reaction of MGO with proteins to form a Schiff-base (“the protein transfer of one of its electrons onto an acceptor of small size and incorporate this acceptor”) was called doping of proteins (Szent-Gyorgyi 1977). The first in vivo glycated protein identified was hemoglobin. Samuel Rahbar screened for hemoglobin variants and found in 1968, a minor “abnormally fast-moving hemoglobin band” in diabetic patients, the subfraction HbA1c. This is now the basis of the procedure for evaluating long-term control of diabetes (Rahbar 2005). Thereafter, glycation was detected in a variety of human tissues and organs and discussed to play a major pathophysiological role during induction of degenerative diseases.
Beside glycation in vivo and their possible pathophysiological role, there were parallel investigations to understand glycation in the context of nutrition (Fig. 1). Depending on the conditions like sugar content, time and temperature, heating of food can induce AGE formation, resulting in low to very high yields of AGEs. A diversity of products are formed in the Maillard reaction, which seem to be important for color and flavor. Therefore, this reaction is important for the food industry. Interestingly, this was known for a long time, for example, it was of interest that the Maillard reaction could be the cause of the browning that occurs during the manufacture and storage of foods (e.g., review by Stadtman ER, Non-enzymatic browning in fruit products, Advances in Food Research 1(1948) 325–372).
The Maillard Reaction: A Short Overview
Within the classical Maillard reaction, a reducing sugar or aldehyde, like glucose, reacts with a free amino group, like the side chain of a lysine or arginine residue, of a protein. The resulting Schiff’s base typically undergoes a rearrangement to form a more stable Amadori product. Further rearrangements, oxidations and eliminations are needed to finally form the members of the highly heterogenous group of AGEs. AGEs are stable at physiological pH, and their rate of accumulation in tissues depends especially on the turn-over rate of the affected proteins (Stitt 2010). Therefore, especially extracellular matrix proteins like elastin and collagen are modified due to their normally long half-life in vivo.
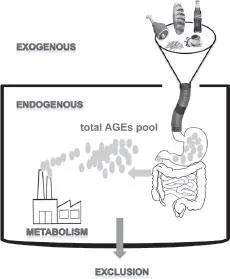
Figure 1A. AGEs not only reach the body with the food intake (exogenously) but are also produced as a result of the metabolism (endogenously). The exogenous and endogenous AGEs build the total AGEs pool in the body. Most of the AGEs are then excluded from the body.
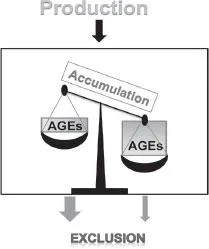
Figure 1B. When there is a misbalance between the production and exclusion (e.g., diseases), AGEs accumulate.
Sugars differ in their ability to react with amino groups. Generally, smaller sugar molecules (oxoaldehydes) are more reactive than sugars with a higher number of carbon atoms (Bunn and Higgins 1981). Reactivity also depends on the proportion of the sugars in the more reactive open chain conformations (Franks 1987). For example, fructose, which is to a higher degree in the open form than glucose, is about 10-fold more reactive in terms of glycation of proteins than glucose. In line, fructose supplemented growth of Saccharomyces cerevisiae cells as compared to growth on glucose resulted in a more pronounced age-related decline in yeast reproductive ability and higher cell mortality. This was mainly explained by the higher reactivity of fructose in glycation and ROS generation in vivo (Semchyshyn et al. 2011). D-ribose participates in protein glycation both inside (endogenous glycation) and outside the body (exogenous glycation). Syrovy studied glycation of albumin using four different reducing sugars. Results showed that D-ribose was more active in glycation than the other sugars used (Syrovy 1994). The glycating ability of reducing sugars seems to increase as follows: D-glucose < D-mannose < D-galactose < D-xylose < D-fructose < D-arabinose < D-ribose < 2-deoxy-D-ribose (Monnier 1990).
Important acceptors of the sugars are amino acids like lysine or arginine or amino groups on nucleobases and lipids. Besides monosaccharides, small α-oxocarbonylic compounds (especially dicarbonyls) contribute significantly to AGE formation as they are far more reactive than sugars (Lo et al. 1994, Thornalley 2007). High levels of these reactive compounds such as 3-deoxyglucosone, glyoxal or methylglyoxal induces the so-called “carbonyl stress”. Different reactions lead to the formation of dicarbonyls, from a byproduct of the glycolysis (methylglyoxal) to a product of lipid peroxidation (glyoxal). Depending on the amino acid sequence of the proteins, specific amino acids can be modified by sugars, resulting in some kind of unexpected specificity (Munch et al. 1999).
Biological Action of Glycated Proteins
AGEs can have impact on the function of biological systems by several means. Modifications on proteins can clearly alter structure, enzymatic activity and biological half-life (Bulteau et al. 2001, Friguet et al. 1994). If DNA is modified, the consequence can be mutations, and if membrane lipids are hit, this might affect transport and signalling processes. Last, but not the least, AGEs can act through specific receptor molecules.
For several enzymes it has been shown that the presence of an AGE-modification alters, if not destroys their activity. Glycation of the Na,K-ATPase in vitro in the presence of ATP, results in a shift in the steady state kinetics of ATP hydrolysis, whereas in the absence of ATP, the enzyme activity is irreversibly inhibited (Garner et al. 1990).
Extracellular proteins are well known targets for AGE-modifications. Proteins such as collagen have a relatively long biological half-life and are, especially in the case of diabetes, directly exposed to high levels of glucose outside the cell. Interestingly, modified collagen becomes more resistant to degradation by MMPs which causes accumulation of AGE-modified collagens in the ECM (Bartling et al. 2007). The occurrence of AGE cross links such as GOLD or MOLD result in stiffening of the ECM, which often compromises organ function and is associated with several chronic diseases such as diabetes, vascular diseases, retinopathy, arthritis and also Alzheimer’s syndrome.
Although glycation seems to be a rather unspecific reaction, it seems that some proteins are prone to becoming the major modified protein in a cell. It is not clear whether this is simply a consequence of exposure of reactive residues on the surface or in the catalytic site of the proteins, or whether it reflects physiological functions such as a protective mechanism. Examples of predominantly modified proteins include several HSPs. In case of HSP-27, a higher anti-apoptotic effect became evident after methylglyoxal modification. In stem cells, the constitutive heat shock protein HSP-70 protein was described as by far the most modified protein (Hernebring et al. 2006). In yeast cells, only a few proteins were found to be modified by methylglyoxal (Gomes et al. 2006, Gomes et al. 2005). These proteins include enolase, phosphoglyceratemutase and aldolase, all involved in glycolysis. Additionally, very similar to mammalians, three heat shock proteins, involved in protein salvage were glycated (HSP 71/72, HSP26). Although enolase was inhibited by methylglyoxal modification, the glycolytic flux was not affected (Gomes et al. 2006) showing that yeast glycolytic machinery is resistant to glycation that results from the high glycolytic activity in these cells.
According to an early hypothesis, the formation of AGE-modifications might have regulatory functions as it reflects physiological states, such as glycolytic activity of cells. This is clearly in contrast to the modern view that focuses on the association of AGE-formation with pathophysiological processes. However, it could be shown that high glycolytic flow caused increased modification of the transcriptional corepressor mSIN3a by methylglyoxal, which resulted in coupling of glycolytic activity to changes in gene expression, namely angiopoetin-2 transcription (Yao et al. 2006, 2007a, Yao et al. 2007b).
The finding that embryonic stem cells contain large amounts of glycated proteins that are rapidly eliminated when differentiation processes occur (Hernebring et al. 2006) showed that glycation per se is not generally deleterious to the cells and may even be involved in maintaining the undifferentiated state.
Cells possess specific binding and receptor molecules for AGEs (Ott et al. 2014). The major and best known receptor for AGEs is the “Receptor for AGEs”, RAGE or synonymous AGER. But other binding proteins have also been described. These are oligosaccharyltransferase (OST48, AGE-R1), 80K-H (AGE-R2) (Li et al. 1996), galectin-3 (AGE-R3) (Vlassara et al. 1995), CD36 (Kuniyasu et al. 2003, Ohgami et al. 2001, Ohgami et al. 2002), and scavenger receptors IIa and −b (Takata et al. 1988, 1989). RAGE is a member of the immunoglobulin receptor family and binds several ligands such as AGEs, HMGB-1, S100 proteins or amyloid beta peptide. Binding of agonists like the AGEs to RAGE results in activation of NADPH-oxidases and other less well described pathways that lead to increased production of ROS. The major downstream target of RAGE is the proinflammatory NFκB-pathway, which in turn leads to elevated RAGE expression and perpetuation of the cellular inflammatory state (Bierhaus et al. 2006). This inflammatory state is characterized by the production of inflammatory cytokines such as IL-6 and TNFα.
AGEs in Aging and Diseases
The presence of AGEs is correlated with age and several important diseases. Only few studies are enumerated as examples. As diabetes is often accompanied with hyperglycemia and oxidative stress, an accelerated rate of AGE-formation is observed. This glycation reaction contributes to morbidity of diabetes, end stage renal and heart diseases, arthritis and cataracts. Immunolocalization of carboxymethyllysin (an AGE compound) in fetal, juvenile, and adult normal tissues (skin, lung, heart, kidney, intestine, intervertebral disc, artery) revealed a clearly age-dependent staining pattern. Only tissues from adults showed significant positive staining and the process was accelerated by diabetes (Ceol et al. 1996). High levels of glycated compounds were found in cerebral, cardiac and renal tissues of patients dead from coronary heart disease, complicated and not complicated from diabetes mellitus and essential hypertension compared to victims dead from traumas. Furthermore, in experimental studies on rabbits and rats, it was shown that the content of glycoconjugates in tissues did not increase in short-term (exogenous or stress-associated) hyperglycemia, while accumulation of glycoconjugates in pericapillary, pericytic, and cellular structures in long-term hyperglycemia caused deformation of...