
- 440 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Rheological Properties of Cosmetics and Toiletries
About this book
This volume in the Cosmetic Science and Technology series covers the important rheological aspects of cosmetic and toiletry formulations, including theoretical physical chemistry, instrumentation and measuring techniques, raw materials and stability predictions. The work discusses the specific rheological requirements of nail polish, antipersirants and deodorants, dentifrices, hair care products, creams and lotions.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Rheological Properties of Cosmetics and Toiletries by Dennis Laba in PDF and/or ePUB format, as well as other popular books in Medicine & Chemistry. We have over one million books available in our catalogue for you to explore.
Information
1
The Flow of Cosmetics and Toiletries
Dennis Laba
RHEOX, Inc.
Hightstown, New Jersey
Hightstown, New Jersey
From atoms to galaxies, everything is in motion. Flowers sway in the wind, blood circulates through our bodies; even the continents “drift.” Motion is an inherent part of the world. To describe these motions, we have come up with a variety of terms: flowing, vibrating, slipping dripping, running, squirting, oozing, seeping. Each term conjures up some specific type of motion and immediately we understand the type of movement described. In fact, our associations are so strong that if during a heavy rain someone were to tell us nonchalantly that there was water “gushing” through the roof (rather than leaking), we would certainly wonder why they did not seem too concerned.
The flow or movement of all materials can also be described by specific equations that have been developed over the years. Gases and solids flow, but usually when we hear the term “flow,” it is used to describe the motion of liquids. It is this liquid flow that we most associate with the term “rheology.” A quick definition of rheology is “the science or study of how things flow.” This definition encompasses everything from the turbulent flow of a waterfall to the slow, drawn-out flow of cold maple syrup.
Cosmetic and toiletry products flow. We may not always be aware of their flow, but nevertheless, they flow. When a hand lotion is pumped through a dispenser, it is flowing. As it is expelled from the dispenser, it must “flow” onto the hands. If it “drips” onto the hand, or “runs off” the hand, there is something wrong with it. The characteristic flow of an elegant, rich-bodied lotion is expected.
The seemingly simple task of applying nail polish is actually Theologically complicated. The pigment suspension must exhibit enough “body” or “viscosity” to be picked up by the brush, yet must be easily transferred to the nail. During application, the lacquer must balance between two opposing needs: the need to be thick, so as not to run off the nail; and the need to be thin, so that the brush strokes from application level out and disappear, leaving a smooth, glossy appearance.
Each cosmetic and personal-care category has its own characteristic rheology. (See Fig. 1.) Many antiperspirants are suspensions and must therefore be formulated with rheology in mind. The main rheological concern of suspensions is that the solids want to settle to the bottom, by forcing the liquids to move out of their way. If the active ingredient does not stay uniformly distributed throughout the product, or is not easily resuspended when shaken, the consumer may apply an improper dosage. This rheology problem can result in the antiperspirant being ineffective (in the case of too little being delivered) or irritating (in the case of too much being delivered).
The application of a stick product, whether an antiperspirant or a lipstick, involves rheology. In the case of a lipstick, the product is expected to transfer easily from the stick to the lips. If this is not accomplished, the coverage on the lips may be uneven and unacceptable. A lipstick is also expected to retain its shape, even under very hot conditions. When a tube is left in a purse, either on the beach or in a hot car, the woman expects the lipstick to stay where it belongs, and not cause a mess by flowing out of the container.
As you can see, formulating chemists must not only concern themselves with the flowing part of rheology, they must also keep in mind those instances where the absence of flow is needed. After a dentrifice is squeezed out of a tube, it must rebuild its viscosity quickly before it sinks into the bristles of a toothbrush. A sunscreen or shampoo is expected to have enough body so that after it is poured from the container, it does not run out of the hand before it is fully applied. Even the foam characteristics of shampoos should be carefully designed so that the thick, rich lather stays on the top of the head and minimizes the amount of surfactants or active ingredients that can drip down into the consumer's eyes and cause irritation.
During the production of cosmetics and toiletries, there are times when rheology becomes critical. While stick products are in a molten state, the pigments or active ingredients can settle quickly to the bottom of the mixing tank. If this happens, the resulting product will not be acceptable. There are several ways to remedy this, involving either reducing the particle size of the solids, carefully controlling the viscosity of the batch through temperature variation, or including what is called a “rheological additive.” These specialized additives can help change the flow characteristics of the batch.
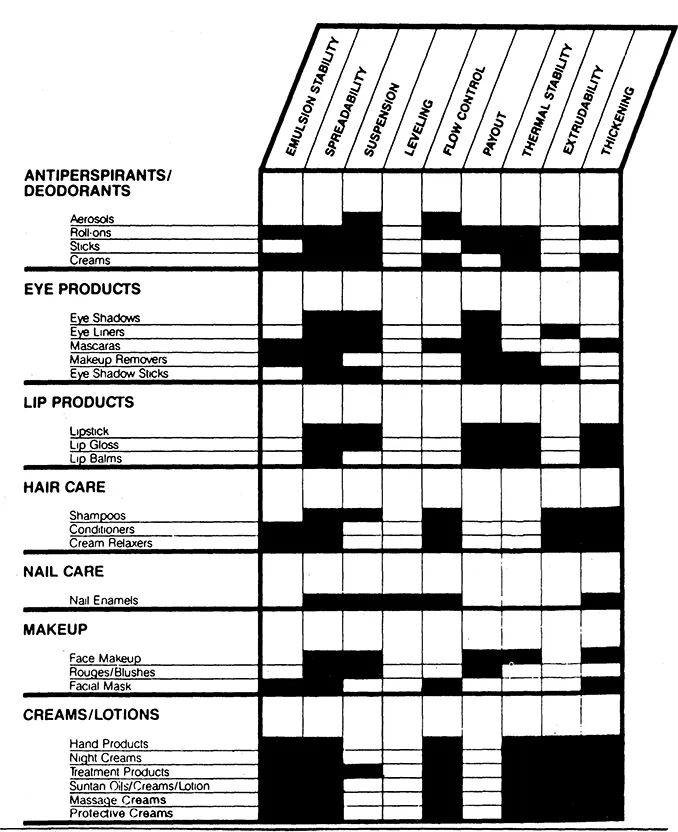
Figure 1 Rheological concerns in commercial products. (Reprinted with permission of RHEOX, Inc.)
We will be better able to discuss these additives if we first briefly define some terms.
As in every science, rheology has its own specialized language. It is not the intent of this chapter to go into detailed descriptions and equations, but a brief explanation of some rheological terms and properties should be helpful.
Viscosity: The most widely used and most familiar rheological term. It is commonly defined as “how thick something is.” In slightly more technical terms, viscosity is “the resistance to flow” and is measured by the ratio of shear stress (force) to shear rate (movement).
Shear stress: A force applied to an area.
Shear rate: The ratio of the velocity of material to its distance from a stationary object. Cosmetic and toiletry products undergo a wide variety of shear rates, ranging from the low shear rate encountered in a lotion being poured from the container to the very high shear rates of brushing on a nail enamel. Some typical shear rates for cosmetic and toiletry products are given in Table 1.
Yield value: The minimum amount of force necessary to induce flow.
Viscosity profile: A graph characterizing the viscosity response of a material to changes in shear rate.
Suspension: The tendency for a solid to remain immobilized in a liquid.
Newtonian flow: The flow best characterized by water. Regardless of the shear applied, its viscosity remains the same.
Pseudoplastic flow: A “shear-thinning” type of flow. As the material is sheared, its viscosity decreases. When the shearing stops, its internal structure (which gave it the original viscosity) rebuilds very quickly.
Dilatent flow: Best characterized by quicksand. With increasing force or shear rate, the viscosity increases. When the force is stopped, the viscosity quickly reverts to its original value.
Thixotropic flow: A time-dependent rheology. Thixotropy starts off similar to pseudoplasticity: with increasing shear rate, the viscosity drops. The difference lies in the recovery speed of the material. While pseudoplastics recover very quickly, full thixotropic redevelopment of the original structure could take from seconds to days. Many lotions are thixotropic. If you try pouring them after the container is shaken, you'll find that they flow much more easily.
Table 1 Typical Shear Rates of Cosmetic/Toiletry Actions

Viscometer: An instrument used to measure viscosity. The viscometer most widely used in this industry is typically called a Brookfield, after the company that manufactures it.
Rheometer: An instrument used to measure the many different facets of rheology. All viscosimeters are also rheometers. Some Rheometers are much more complicated (and costly) than simple viscometers, but are capable of providing much more information in a variety of ways. For example, a rheometer may be able to run an entire shear rate range automatically, from 10-3 s-1 to 103 s-1, periodically taking viscosity readings and providing a viscosity profile.
The task of formulators very often involves overcoming natural tendencies. They are typically asked to suspend solids that naturally want to settle, stabilize mixtures of two liquids which want nothing more than to separate from one another, and produce materials that are thick at some times and thin at other times. Luckily, formulators have quite an arsenal of materials that can be used to help them achieve their goals. As a group, these ingredients are called “rheological additives.”
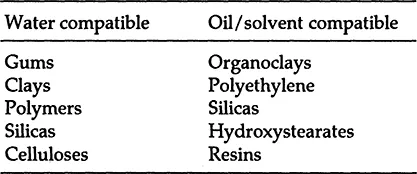
There are many different ways to classify rheological additives. The most general classification is based on whether or not the additive is compatible with water.
For convenience, we will call the two classes “water compatible” and “oil/solvent compatible.” Table 2 gives examples of rheological additives in both categories.
Of course, within each type are a number of different variables, each giving a slightly different rheological response. Formulating chemists need to have a working knowledge of rheological additives so they can efficiently achieve rheological control of their system. Some of the additives can form stiff gels at 0.5%, while others are still flowable in certain systems up to 10% concentration. Some additives thicken as soon as they are added, while others need to be neutralized, sheared, or thermally activated. Certain additives can be used to make clear systems, while others can impart heat stability, emulsion stability, or suspension.
Table 2 Rheological Additives
Once a formulator has successfully worked his or her way through the maze of rheological additives, the job is still not over. For a product to be viable in the marketplace, it must also be stable. Stability testing must be carried out to assure that the product will not degrade before the consumer has a chance to use it. Subtle changes on the supermarket shelf can lead to big trouble if the product becomes unusable. Even a slight degradation in the esthetics of the product could be enough for a consumer to decide not to rebuy that particular brand. Along with checking the physical, microbial, and chemical properties, many of the rheological characteristics are also followed in an extensive stability program: viscosity; suspension; leveling; extrudability; and spreadability. A battery of standardized as well as “in-house” tests are used. Much of the testing involves sophisticated instruments, but no instrument yet can replace the use of the human being.
All the nuances of human touch are lost on a machine. Human sensitivity to rheological properties such as viscosity, slip, drag, and tackiness is very acute. At the same time as he or she is making mental readings of these properties, a person can also be getting visual input concerning appearance, color, gloss, and flow characteristics. Other tactile sensations which maybe automatically registered include the product's temperature, wetness, oiliness, greasiness, grittiness, silkiness, and firmness. The olfactory sense will also kick in to determine a level of contentment with the fragranced (or unfragranced) product. The ears will pick up and register any sounds made as the product comes through a pump or aerosol, and if the product is used in the mouth the taste buds will immediately let us know how they like it and store away that particular sensation.
Instruments may be able to exceed human perception, but so far no one has figured out how to blend all the different instrument readings for all the different properties and come up with a definitive answer as to exactly how esthetically pleasing a product will be. Many attempts have been made to characterize rheologically pleasing products instrumentally, and great strides have been made, but for the final analysis, we must still rely on a “human rheometer.”
Bibliography
1. Philip Alexander, “Rheology Principles Measurement and Control,” Manufacturing Chemist (April 1986).
2. Philip ...
Table of contents
- Cover
- Half Title
- Title Page
- Copyright Page
- Table of Contents
- Preface
- Contributors
- Chapter 1 The Flow of Cosmetics and Toiletries
- Chapter 2 Introduction to Rheology
- Chapter 3 Instrumentation
- Chapter 4 Rheological Additives
- Chapter 5 Nail Product Rheology
- Chapter 6 AntiperspirantIDeodorant Rheology
- Chapter 7 Dentifrice Rheology
- Chapter 8 The Rheology of Hair Products
- Chapter 9 Emulsion Rheology: Creams and Lotions
- Chapter 10 Predicting Stability in Rheologically Modified Systems
- Chapter 11 Rheological Profiles
- Index