
- 280 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Hydrometallurgy in Extraction Processes, Volume II
About this book
This two-volume set provides a full account of hydrometallurgy. Filled with illustrations and tables, this work covers the flow of source material from the mined or concentrate state to the finished product. It also highlights ion exchange, carbon adsorption and solvent extraction processes for solution purification and concentration. The extensive reference list-over 850-makes this set a valuable resource for extraction and process metallurgists, researchers, and practitioners.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Hydrometallurgy in Extraction Processes, Volume II by C. K. Gupta,T. K. Mukherjee in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Chemistry. We have over one million books available in our catalogue for you to explore.
Information
Chapter 1
OTHER LEACHING PROCESSES
I. GENERAL
In addition to the specific leaching processes described in the previous volume, there are also a number of others which are of considerable importance in the field of hydrometallurgy. Among such miscellaneous processes, special mention may be made of (1) cyanide leaching, (2) chlorine leaching, (3) hypochlorite leaching, (4) hypochlorous acid leaching, (5) dichromate leaching, and (6) electrochemical leaching. This chapter has been organized to provide an account of these leaching processes.
A. Cyanide Leaching
Processing of gold and silver ores by cyanide leaching is one of the prominent examples of early hydrometallurgy-based processes realized on a commercial scale. Today, one finds the cyanide leaching process as successfully taken and applied to other areas. In this context, one may cite as a major example the successful application of cyanide leaching to sulfidic resources of copper. Elaboration of this particular application area of cyanide leaching appears in a later text of this section.
1. Basics of Cyanide Leaching of Au and Ag Ores
Gold and silver readily dissolve in dilute cyanide solutions and this fact was known almost two centuries ago. The reason, however, remained unexplained for quite some time. It was not understood why noble metals like Au and Ag could be so easily leached in dilute cyanide solutions and also why rates of leaching showed a dependence on cyanide concentrations only up to a certain critical limit. In 1846, a publication from Elsner1 proposed for the first time that Au dissolved in cyanide solution only in the presence of O2 according to following reaction
(1) |
Dissolution of Ag in cyanide solution can be similarly shown as:
(2) |
More than 40 years later, Janin2 suggested that dissolution of Au and Ag took place not by reduction of O2, but by liberation of H2
(3) |
(4) |
The support for the leaching reaction (1) as proposed by Elsner came from Bödlander.3 He, however, pointed out that the reaction proceeded in two stages as shown here
(5) |
(6) |
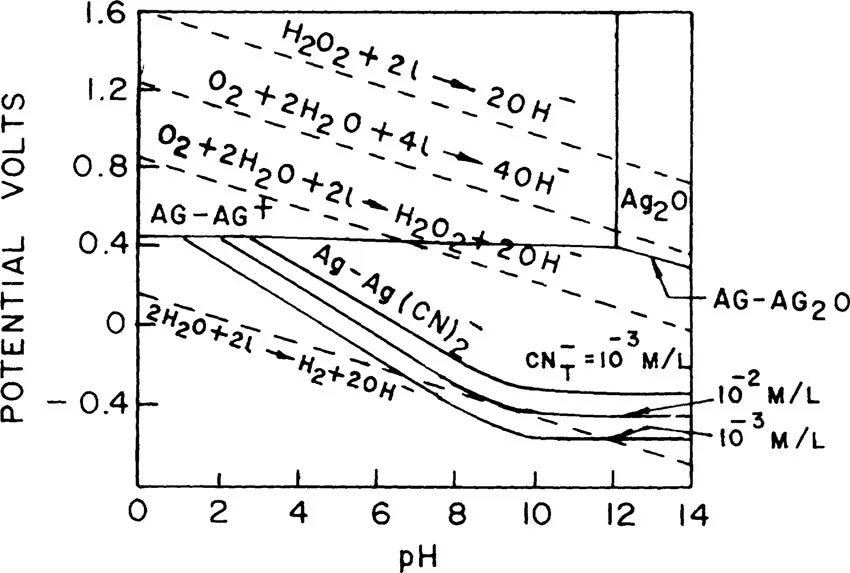
FIGURE 1. Potential-pH diagram for Ag-H2O and Ag-H2O-CN, Ag-10−6 mol/1; H2O2-10−6 mol/1; temperature − 25°C.
(7) |
(8) |
Other investigators4, 5 thought that intermediate agents like cyanogen gas [(CN)2] and potassium cyanate (KCNO) were responsible for the dissolution of Au and Ag. Such assumptions were, however, later proved6, 7, 8 to be wrong. Deitz and Halpem9 suggested that all these reactions could basically be considered as oxidation and reduction steps. The oxidation step can be represented as
(9) |
and the reduction step as
(4a) |
(2a) |
(7a) |
(8a) |
The potential-pH diagrams for Ag-H2O and Ag-H2O-CN− are shown in Figure 1. This diagram shows that in the absence of cyanide, an insoluble Ag2O phase is formed at a pH higher than 12.3. The equilibrium potential between Ag and Ag+ is 0.446 V. The addition of cyanide lowers the concentration of free Ag+ ions through the formation of argentocyanide complex as given in the equation
(10) |
and allows the Ag2O phase to disappear. These findings are further summarized in Figure 2, which presents the potential-pH diagram for various dissolution reactions of silver in aqueous cyanide. It can be seen that Equations 2a, 7a, and 8a correspond to positive potentials(i.e., negative free energy change) and are therefore thermodynamically feasible, whereas Equation 4a is unfavorable because it is associated with negative potential. Barsky et al.10 determined the equilibrium constants for these reactions and arrived at similar conclusions regarding feasibilities of these reactions. Based on experimental observations, Kudryk and Kellogg11 argued that the product of the reduction of oxygen was hydroxyl ions and not hydrogen peroxide. But Kameda12 and later Lund13 showed that for every 2.2 equivalents of metal dissolved, 1 mol of O2 was consumed and 1 mol of H2O2 was produced. Similarly, for every 1 equivalent of metal dissolved, 2 mol of cyanide were consumed. Based on these findings, Habashi14 pointed out tha...
Table of contents
- Cover
- Title Page
- Copyright Page
- Table of Contents
- Chapter 1: Other Leaching Processes
- Chapter 2: Solution Purification
- Chapter 3: Metal Recovery Processes
- Index