
- 404 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Extractive Metallurgy of Molybdenum
About this book
Extractive Metallurgy of Molybdenum provides an up-to-date, comprehensive account of the extraction and process metallurgy fields of molybdenum. The book covers the history of metallurgy of molybdenum from its beginnings to the present day. Topics discussed include molybdenum properties and applications, pyrometallurgy of molybdenum, hydrometallurgy of molybdenum, electrometallurgy of molybdenum, and a survey of molybdenum resources and processing. The book will be a useful reference for metallurgists, materials scientists, researchers, and students. It will also be an indispensable guide for world producers, processors, and traders of molybdenum.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
Chapter 1
PROPERTIES AND APPLICATIONS OF MOLYBDENUM
I. INTRODUCTION
Molybdenum, pronounced “mo-lyb-den-um” and often abbreviated as “moly”, is a metal that is gaining increasing significance in our industrial world. The early history of the metal was shaped by two scientists, Carl Wilhelm Scheele and Peter Jacob Hjelm. It was Scheele who in the year 1778 demonstrated that molybdenite, the principal molybdenum mineral, was a discrete mineral sulfide. After over 3 years, late in 1781, Hjelm successfully isolated the metal. He accomplished this by thermally treating molybdic acid with carbon. The next 100 years or so constituted a rather inactive period in the history of molybdenum. It was not until 1893 that the metal was finally obtained in a relatively pure state by German chemists, who reduced calcium molybdate with carbon and removed the lime with hydrochloric acid. The resultant product was 96% pure metal. The rather impure metal was reported to have been used experimentally as a substitute for tungsten in tool steels at that time. The first recorded application of molybdenum as an alloying element in steel came the following year, when a molybdenum-bearing armor plate was produced in France. Very soon thereafter, the French chemist Henri Mossian succeeded in producing a 99.98% pure metal by car-bothermic reduction of oxide in an electric furnace. It was he who established the atomic weight and many physical and chemical properties of molybdenum.
A chronology of the historical development of molybdenum is presented in Table 1. The first mine production of molybdenum was undertaken in the Knaben mine in southern Norway around the close of the 18th century. Since very little commercial use of the metal existed in those days, the output from the mine remained insignificant until around 1880. By the end of the 19th century, molybdenum ores were also mined periodically in Australia and the U.S. The First World War brought about an appreciable demand for molybdenum, when it was substituted for tungsten in high-speed steels and was also used as an alloying element in certain steels for military armament. The end of the war in 1918 very adversely affected the molybdenum market. The production of armaments and of such implements of war as tanks, armored cars, heavy guns, and warships were drastically scaled down. A substantial stock of molybdenum was left behind, since at that time the metal had only very limited application for nonmilitary purposes. Thus, the period 1912 to 1920 was a very lean period for molybdenum. Almost all molybdenum mine production was tapered off. This period, however, was important in two ways. The flotation process for the recovery of molybdenite was developed and an intensive research effort went into the exploration and development of peaceful uses of molybdenum. These efforts established the suitability of the metal in many peacetime applications, primarily as an alloying addition in steels and cast irons. As a consequence, the demand for molybdenum picked up and, in fact, exceeded that registered during the war years. The molybdenum mines in the U.S. resumed operations and from 1924 onwards the use of molybdenum grew steadily. The Second World War brought another spurt in the demand for molybdenum. By this time, molybdenum had acquired the distinction of being a very useful material in the metallurgical industry. During the postwar period, many new technological applications were developed for molybdenum and its alloys. The progress particularly in the peaceful uses of molybdenum had been phenomenal, and with all this, its military uses, although not of strategic importance, decreased. Molybdenum is now a vital strategic material in the world economy, not only because of its military uses, but also because of its extensive industrial applications. The resource distribution and the production of molybdenum in the world are quite uneven. After more than two centuries since its discovery in 1778, and with about more than half a century of effective technological uses, molybdenum itself has been established as an important additive metal in respect of a wide variety of alloys. It is quite certain that this metal will see progressively increasing applications in years to come.
TABLE 1
A Chronology of the History of Molybdenum
Sequence no. | Year | Major event |
1. | 1778 | Discovered by Carl Wilhelm Scheele |
2. | 1781 | Peter Jacob Hjelm produced molybdenum powder |
3. | Near the end of the 18th century | First production of molybdenite from Knaben mine in Norway |
4. | By the end of 19th century | A small production developed in Australia and the U.S. |
5. | 1898 | First attempt to use molybdenum as an alloying constituent in steels |
6. | 1906 | W.D. Coolidge filed a patent for rendering molybdenum ductile |
7. | 1913 | Frank E. Elmore developed vacuum flotation cell to recover molybdenite from ores in Norway |
8. | First World War | Serious deficiency in the supply of tungsten which forcibly introduced molybdenum as a substitute for tungsten |
9. | End of First World War in 1918 | Molybdenum demands slumped — led to closure of all molybdenum-producing mines, Climax and Questa among the principals |
10. | 1912—1920 | Almost all countries, with the exception of Australia, suspended moly production; the period is marked by intensive research into peaceful uses of molybdenum; the flotation process for separating molybdenite from its associated ores developed |
11. | 1920 | Studebaker started using low alloy molybdenum bearing steels in automobiles |
12. | 1923—1924 | The application of molybdenum-bearing steels for the automobile industry was established; reactivation of molybdenum mines in the U.S.; Questa mine in New Mexico was reopened in 1923 and the Climax mine in Colorado started its production in 1924; from this period onward there was a steady growth in molybdenum output, with the U.S. predominating in world production |
13. | 1924—1930 | Brainerd Phillipson diversified and promoted the applications of molybdenum in peace-time uses and was considered as a founder of the molybdenum industry |
14. | 1933 | First byproduct recovery from porphyry coppers at Cananea in Sonora, Mexico (a subsidiary of Anaconda) by Guy Ruggles |
15. | Second World War | An estimated amount of 170,000 metric tons of molybdenum was consumed for all the necessary military and civil hardware for the war between 1938 and 1945 |
This short account of the history of molybdenum will now be followed by a description of the physical, chemical, and mechanical properties of molybdenum, its applications, and its various important alloys and compounds. Short sections on metallography and the identification of molybdenum have also been included.
II. PROPERTIES
A. PHYSICAL PROPERTIES1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17
Molybdenum has an atomic number 42, an average atomic weight of 95.95, belongs to the sixth group of the periodic system of elements, and occurs between chromium and tungsten vertically and between niobium and technetium horizontally (Figure 1). It has two incomplete outer rings of electrons (rings N and O). The distribution of the electrons in the incomplete ring N is 4s2, 4p6, and 4d5, and that in O is 5s1. The most stable valence state of molybdenum is 6. The lower and less stable states are 5, 4, 3, 2, and 0. An important property of molybdenum is its low thermal neutron capture cross-section, which makes it suitable as a structural material for use in the nuclear reactor core. Molybdenum has seven stable isotopes with masses, 92, 94, 95, 96, 97, 98, and 100 in proportions that vary from 9.04% (Mo94) to 23.78% (Mo98). The atomic radius of Mo is 1.39 Å and the ionic radius of Mo4+ is 0.68 Å and of Mo6+ is 0.65 Å.
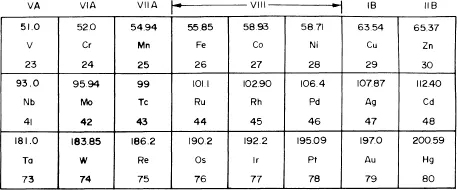
FIGURE 1. Relative position of molybdenum in the periodic table.
In its pure state, molybdenum is a lustrous gray malleable metal, capable of being filed and polished. It can also be turned and milled without difficulty. Molybdenum is an important refractory metal with a very high melting point (~2610°C). Only carbon, tungsten, rhenium, tantalum, and osmium possess still higher melting points. Its boiling point is 5560°C and its density is 10.22 g/cm3 at 20°C. The coefficient of thermal expansion is about one third to one half that of most steels. At elevated temperatures, this low expansion provides dimensional stability...
Table of contents
- Cover
- Title Page
- Copyright Page
- Foreword
- Preface
- Table of Contents
- Chapter 1. Properties and Applications of Molybdenum
- Chapter 2. Resources and Processing
- Chapter 3. Hydrometallurgy
- Chapter 4. Pyrometallurgy
- Chapter 5. Electrometallurgy
- Index
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Extractive Metallurgy of Molybdenum by C. K. Gupta,C.K. Gupta in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Industrial & Technical Chemistry. We have over one million books available in our catalogue for you to explore.