
- 708 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
Explaining principles essential for the interpretation of data and understanding the real meaning of the result, this work describes carious methods and techniques used to characterize dispersions and measure their physical and chemical properties. It describes a variety of dispersions containing particles ranging from submicron sizes to aggregates and from hard particles to polymer latices.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Dispersions by Erik Kissa in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Chemistry. We have over one million books available in our catalogue for you to explore.
Information
1
Dispersion: The System
I. Terminology
A disperse system consists of fine insoluble or only slightly soluble particles distributed throughout a continuous medium. The particles distributed within the continuous phase constitute the dispersed phase, i.e., the internal phase. The continuous phase, or the external phase, is termed the dispersion medium or the dispersion phase.
The dispersed phase, and the continuous phase as well, may be a gas, a liquid, or a solid (Table 1.1).
It may be confusing that all disperse systems, consisting of gas, a liquid, or a solid dispersed in a gas, liquid, or solid, are sometimes termed dispersions while the term dispersion is used to describe specifically a disperse system consisting of a solid dispersed in a liquid medium. The latter definition of the term dispersion is used in this book, which is focused on systems of solid or semisolid particles dispersed in a liquid medium. The dispersions so defined include a broad spectrum of technologically and biologically important systems, such as paints, inks, dyes, concrete, metal particles, detergents, and—last but no means least—the most important dispersion: blood.
A distinction between the terms dispersion and suspension is vague and not universally accepted. According to the IUPAC terminology, a dispersion is colloidal if at least one dimension of the dispersed particles is between 1 mμ and 1 nm [1]. If the particles are larger than 1 μm, the system is usually termed a suspension, although the differentiation is usually imprecise. A concentrated solid-in-liquid system of nonbuoyant coarse particles is called a slurry.
According to the terminology recommended by IUPAC the two-phase solid-in-liquid systems are dispersions when the particles have colloidal dimensions, and suspensions when the particles exceed colloidal dimensions, while still being buoyant. Unfortunately, the distinction between dispersions and suspensions in accord with this definition requires an exact information on particle dimensions, which is not always available. Some authors have used both terms interchangeably in the same article. For example, in a recent article the term colloidal suspensions has been used [2]. In this book, dispersion is used as the generic term. However, the term used by the author of an article cited is repeated without attempting to distinguish between the terms dispersion and suspension.
TABLE 1.1 Disperse Systems
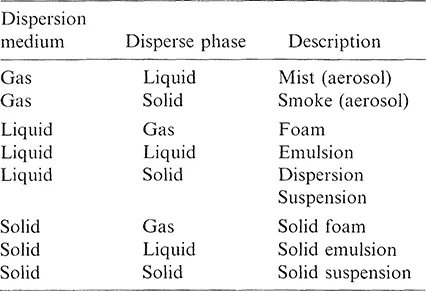
A colloidal dispersion whose dispersed phase is nonpolymeric in nature is termed a sol. If the dispersion medium is a gas, the sol is termed as an aerosol, if the dispersion medium is a liquid, the sol is called a lyosol; and if the dispersion medium is a solid, the sol is described as a solid sol. If the dispersed phase is a polymer, the dispersion is called a latex. The distinction between the terms “emulsion” and “dispersion,” the latter denoting a dispersed system containing a solid dispersed phase, is sometimes vague. For example, a photographic emulsion consists of solid silver halide particles distributed in gelatin [3].
Dispersibility is defined as the ease with which a dry powder may be dispersed in a liquid medium. Substances that facilitate dispersing or stabilize dispersions are termed dispersants.
Dispersions are defined as stable or unstable. Dispersions are stable if the number of particles within a unit volume of the liquid medium remains constant during storage for a long time, e.g., several months or years. The stability may have a thermodynamic or kinetic basis. In an unstable dispersion the number of particles may decrease with time, because of flocculation (Chapter 8) or sedimentation (Chapter 9). The settling of the particles and the formation of a sediment is termed sedimentation. The forces acting upon a dispersed particle are a gravitational force, a Brownian force, and interaction with other dispersed particles. Small dispersed particles are kept suspended in the dispersion medium by forces of a random thermal movement, described as the Brownian motion. If the particles are small, of colloidal dimensions, and their density is not significantly different from that of the dispersion medium, the effect of gravitational forces may be negligible and the thermal agitation of the Brownian motion is sufficient to keep the particles dispersed. If the particles are larger, however, and have a density much larger than that of the dispersion medium, the gravitational forces can be considerable and cause settling of particles to the bottom in the liquid. The rate of sedimentation is the amount of the dispersed solid settled in a unit time interval. The volume of the sediment is called the sedimentation volume.
The Brownian motion inevitably causes collisions of the moving particles. In a stable dispersion particles collide with the molecules of the dispersion medium or dispersants and remain discrete particles. In an unstable dispersion the Brownian motion brings particles together to a close contact and forms aggregates, termed flocculates or coagulates. The terminology used to describe the aggregation of particles is far from uniform. Flocculation has been defined as the process by which dispersed particles form aggregates termed floes [4]. Aggregation of particles increases the sedimentation rate. The gravitational forces acting upon the aggregate increase with the number of particles in the aggregate, whereas the resistance to settling increases with the effective radius of the aggregate and consequently with the cube root of the number of particles in the aggregate [5]. A dispersion may flocculate partially before sedimentation or during sedimentation. Flocculation affects the sedimentation volume. In the absence of flocculation the particles slide past each other and form a dense sediment. In contrast, flocculated particles stick together and form a voluminous sediment, the floe [6].
Some authors use the terms flocculation and coagulation synonymously, whereas others distinguish between the two terms [7]. According to IUPAC nomenclature the terms aggregation, coagulation and flocculation represent different processes. As suggested by La Mer [8], coagulation is an aggregation process that forms a coagulum, an aggregate of particles in close contact. Flocculation produces a looser form of an aggregate with a relatively open structure. The difficulty with this terminology is that the structure of an aggregate is rarely known or quantitatively measured [7]. The theoretical difference between the two processes is the origin of the interparticle attraction. Napper [7] has used the term coagulation to describe aggregation induced by van der Waals forces between the colloidal particles. The term flocculation was reserved to particle aggregation caused by polymeric species and the term coagulation to aggregation initiated by a low molecular species, such as an electrolyte. However, Gregory [9] has used flocculation as a generic term for the aggregation process, including polymer-induced aggregation. The term coagulation was used to denote the first step in a water treatment process involving precipitation reactions.
Flocculation of an intrinsically stable dispersion can be kinetically treated as a two-step process: first, destabilization of the particles, and second, collisions between the particles forming the aggregate. Mühle [10] has used the term coagulation to describe a destabilization process involving interparticle attraction as a consequence of double-layer compression or specific counterion adsorption.
Hann [11] has defined flocculation as a relatively reversible aggregation usually associated with the secondary minimum of a potential energy curve. Particles are attached to each other loosely and the surfaces of adjoining particles are separated considerably. Hann termed coagulation as a relatively irreversible aggregation usually associated with the primary minimum of a potential energy curve for two approaching particles. Particles in a coagulum are held together closely.
The definitions given by Hann are in accord with those of La Mer and with the meaning of the latin words “coagulare” and “floccus.”
The procedures employed for the characterization of a dispersion depend on the specific objective of the investigation. Usually the parameters examined are either of theoretical interest or affect dispersion properties essential for the application and use of the dispersion. Since dispersions are complex systems a complete characterization of all parameters is rarely attempted.
II. Dispersed Phase
A. Characterization of Particles
The dispersed phase in a liquid dispersion medium may be a gas, liquid, or solid. This book is mainly concerned with dispersions of solid particles, including particles of polymeric and noncrystalline materials. The term particle has been defined as a discrete entity of solid matter in a dispersed state with a diameter at or below 50ηm [12]. This upper size limitation of this definition is roughly the smallest size of particle visible to the unaided eye. Other definitions have extended the upper size limit arbitrarily to 1000 ηm, above which the particle becomes a granule.
An enormous variety of substances has been dispersed, differing in their chemical composition and physical properties as well. Solid particles that have been dispersed in a liquid medium include a large variety of chemically different materials: metals (e.g., gold, iron), oxides (e.g., silica, aluminum oxide, iron oxide, titanium oxide), inorganic salts (e.g., barium titanate), carbon, and numerous organic compounds (e.g., dyes, organic pigments, polymers). The dispersed solid may be a complex mixture of chemically different solids, e.g., clay. The physical properties of the solid particles, such as size, shape, and surface, can vary largely as well, even within the same dispersion. A typical dispersion is polydisperse; the dispersed particles usually have different sizes and shapes. Dispersions are therefore complex systems requiring a variety of physical and chemical tools for their description.
Groves [12] has noted that the term particle analysis is being gradually displaced by the term particle characterization. This trend s...
Table of contents
- Cover Page
- Halftitle Page
- Title Page
- Copyright Page
- Contents
- Preface
- 1. Dispersion: The System
- 2. Particle Characterization
- 3. Physical Surface Characterization
- 4. Chemical Characterization of Particle Surfaces
- 5. Wettability
- 6. Stabilization with Dispersants and Dispersibility
- 7. Preparation and Testing OE Dispersions
- 8. Elocculation and Coagulation
- 9. Sedimentation
- 10. Microscopy
- 11. Radiation Scattering
- 12. Chromatography
- 13. Electrochemical Methods
- 14. Rheology
- Index