
eBook - ePub
Advances in Microwave Chemistry
- 522 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Advances in Microwave Chemistry
About this book
Advances in Microwave Chemistry discusses the novel bond formation methodologies, synergistic effects of microwaves with other entities, sample preparation including digestion, combustion, and extraction techniques, as well as selectivity in chemical processes. Recent updates are provided on microwave-assisted syntheses of pharmacologically significant aza-, oxo- and other heterocycles, including lactams, nucleosides, bile acids and sterols, the preparation of nanomaterials, composites, and absorber layer materials for thin film.
This book also incorporates comparative discussions involving microwave irradiation with conventional methods in different aspects of organic, inorganic, medicinal, and green chemistry.
Key Features:
- Provides a comparative discussion on microwave irradiation with conventional methods in different aspects of organic, inorganic, medicinal, and green chemistry
- Presents recent applications of microwave radiation in biocatalysis
- Offers a complete package correlating various aspects of microwaves in organic syntheses, the biological impact of products formed in reactions, pharmacological features, and environmental sustainability of the procedures
- Explains microwave-induced reactions on structurally complex bile acids and sterols
- Stands as a valuable and unique addition to the well-established book series, New Directions in Organic and Biological Chemistry
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Advances in Microwave Chemistry by Bimal K Banik, Debasish Bandyopadhyay, Bimal K Banik,Debasish Bandyopadhyay in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Chemistry. We have over one million books available in our catalogue for you to explore.
Information
1 | Microwave Radiation in Biocatalysis |
David E. Q. Jimenez, Lucas Lima Zanin, Irlon M. Ferreira, Yara J. K. Araújo, and André L. M. Porto
Contents
1.1 Introduction
1.1.1 Principles of Microwave Radiation
1.1.2 Influence of Microwave Radiation on Enzymes
1.2 Application of microwave radiation in biocatalysis
1.2.1 Use of Isolated Enzymes in Biocatalysis under Microwave Radiation and Conventional Heating
1.2.2 Biocatalytic Reactions Using Whole Cells of Microorganisms under MW
1.3 Conclusions and perspectives
Acknowledgments
References
1.1 Introduction
Although microwave ovens manufactured for homes have been used since the 1970s, the first report that these energy sources were appropriately being used to accelerate organic reactions was in 1986. In their pioneering studies, Gedye [1] and Guiguere [2] used the domestic microwave as a tool for conducting organic reactions. In these studies, the authors described the results obtained in esterification reactions and cycloaddition with a domestic microwave apparatus [3].
The risk associated with the flammability of organic solvents and the lack of available systems to control temperature and pressure were the main reasons for using microwave reactors developed especially for organic synthesis. Today, this device is safe and allows the synthetic organic chemist control over all reaction parameters (temperature, pressure and power), thus achieving greater reproducibility and safety in the experiments [3, 4].
In the last decade, microwave radiation has been used to simplify and improve the reaction conditions of many classic organic reactions. Reactions carried out under microwave radiation are generally faster and cleaner and have better yields than reactions performed under conventional heating in similar conditions [5, 6]. The microwave methods provide an efficient and safe technology, according to the principles of “Green Chemistry” [7], because this technique enables solvent-free reactions to be performed, decreasing the number of competing side reactions, increasing the yield and reducing the reaction time [8–10].
More recently, microwave radiation became an important tool for performing biocatalytic reactions. The potential of this technique has been exploited, particularly in the resolution of racemates to obtain enantiomerically pure compounds using immobilized lipases [11–14].
The organic synthesis presents a great contribution to obtain molecules with biological activities. Thus, the development of methodologies that apply the principles of Green Chemistry in the synthesis of new selective products with chemo-, regio- and enantio-selective and environmentally benign characteristics is required. Therefore, the use of microwave radiation in synthetic protocols has been very advantageous because the reactions are performed in a very short time in the absence of organic solvents and with a low consumption of energy [15].
1.1.1 Principles of Microwave Radiation
Microwaves are electromagnetic waves like energy carriers; these are located in the region of the electromagnetic spectrum between infrared light and radio waves in the frequency range between 300 and 300,000 MHz (Figure 1.1) [3, 7].
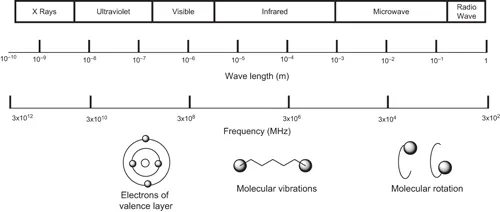
FIGURE 1.1 Illustration of electromagnetic spectrum. (Adapted from Young DD, Nichols J, Kelly RM, Deiters A (2008) Microwave activation of enzymatic catalysis. Journal of the American Chemical Society 130: 10048–10049.)
Domestic microwave ovens operate at a frequency of 2450 MHz (wavelength of 12.24 cm) to avoid interference with frequency telecommunications and mobile phones. According to the Federal Communications Commission (www.fcc.gov), only four frequencies are reserved for Industrial, Scientific and Medical (ISM) purposes: 915 ± 25, 2450 ± 13, 5800 ± 75 and 22,125 ± 125 MHz, with the most commonly used frequencies being 915 and 2450 MHz.
Microwave ovens that can process a frequency change of 0.9 to 18 GHz have been developed for the transformation of materials [16–18].
The microwave is a type of non-ionizing radiation capable of causing molecular motion in dipolar polarization and ionic conduction, but not changes in the molecular structure of molecules [11]. Since the energy of a microwave photon in this frequency region is 0.037 kcal.mol−1, very low energy is needed to break a chemical bond, which is generally of the order of 80–120 kcal.mol−1 [19].
The conventional heating process is fundamentally different to microwave heating upon radiation. In conventional heating an external power source reaches the walls of the flask and heat is transferred from the flask surface into the solution (reagents and solvents); through the driving process, such heating can often cause a convection current in solution. In contrast, with heating under microwave radiation, the energy is transferred directly to the substances by molecular interaction with the ions dissolved in the solution and/or the solvent; thus localized overheating of the substance absorbs the microwave (Figure 1.2) [16, 17]. This type of heating will depend on the ability of that particular material, reagent or solvent to absorb the microwave energy and convert it into heat [19].
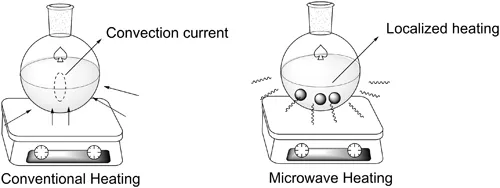
FIGURE 1.2 Illustration of conventional and microwave heatin...
Table of contents
- Cover
- Half-Title
- Series
- Title
- Copyright
- Contents
- Preface
- About the Editors
- List of Contributor
- Chapter 1 Microwave Radiation in Biocatalysis
- Chapter 2 Tracking Microwave-Assisted Sample Preparation Through the Last Years
- Chapter 3 An Insight into Green Microwave-Assisted Techniques
- Chapter 4 Functional Rare Earth-Based Micro/Nanomaterials
- Chapter 5 Microwave-Assisted Synthesis and Functionalization of Six-Membered Oxygen Heterocycles
- Chapter 6 Selectivities in the Microwave-Assisted Organic Reactions
- Chapter 7 Microwave-Assisted Hirao and Kabachnik–Fields Phosphorus–Carbon Bond Forming Reactions
- Chapter 8 Microwave Synthesis of Materials for Thin-Film Photovoltaic Absorber Layer Application
- Chapter 9 Microwave-Assisted Transition Metal-Catalyzed Synthesis of Pharmaceutically Important Heterocycles
- Chapter 10 Microwaves in Lactam Chemistry
- Chapter 11 Microwave Synthetic Technology
- Chapter 12 Microwave-Assisted Green Chemistry Approach
- Index