
- 384 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Hair and Hair Care
About this book
Reviews the chemical and physical properties, care and treatment of hair, including product development. The book discusses ethnic hair, its appropriate management measures and products; emphasizes manufacturing and sales strategies for domestic and international product viability; surveys instrumental methods for product performance evaluation; presents sensory and market research techniques for optimum consumer satisfaction; and more.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
1
Morphology and Properties of Hair
I. INTRODUCTION
Hair fibers form a major component of the outer covering for most mammals. They create a physical barrier between the animal and its environment, and have evolved as a result of their necessary exposure to harsh conditions and the need to be stable over long periods of time to quite severe treatment. All mammalian hairs, together with wools, horns, claws, nails, and quills, mainly consist of a protein material known as α-keratin. As a protein, alpha keratin is a biological polymer consisting of polypeptide chains formed by the condensation of amino acids. In the general formula for a polypeptide chain,
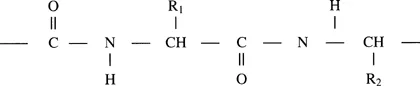
the groups R1 and R2 are the side chains of the amino acid residues for α-keratin corresponding to 20 different compositions. The distinguishing feature of all keratins and the factor basic to the stability of their structure is the presence of a large proportion of the sulfur-containing diamino acid cystine. With its two amino and two carbonyl groups cystine can form part of two adjoining polypeptide chains forming via the disulfide bond of the cystine residue a covalent crosslink between the two chains. Such covalent crosslinks form part of some 10% of the residues of the keratin structures, confirming a high degree of physical and chemical stability to the fiber. In particular in the hair setting process, it will be noted that the disulfide crosslink plays a basic role. Destabilization of the disulfide bond is a key step in most commercial setting procedures.
X-ray diffraction studies of ordered structures such as crystalline solids reveal the spatial repeats and the nature of the molecules forming the repeats. This technique has been applied to the study of hair fibers and indicates a high degree of order (crystallinity) present in the keratin structure. The a in “α-keratins” refers to the typical high-angle x-ray diffraction pattern obtained from hair fibers. Under low-resolution conditions in a dry environment two major reflections are obtained, which are diagnostic for α-keratins. The 0.516-nm reflection on the meridian corresponds to a repeat in the fiber direction and the 0.94-nm reflection on the equator corresponds to spacing repeat at right angles to the fiber direction (1). This latter equatorial reflection is usually quoted at 0.98 nm, this value being based on x-ray tests on fibers carried out at room humidity with no correction for the presence of water (2,3). The α-helix proposed by Pauling to describe the protein molecules forming the ordered regions of the keratin structure was based on this x-ray data taken together with other relevant information (4). Other specialized forms of keratin exist such as “feather-” and “β-keratin.” Both of these forms produce a distinctly different high-angle X-ray diffraction pattern, of which only the (β-keratin as the result of extension of the α-keratin structure will be discussed as being of direct interest in this work.
Hairs are produced completely within the hair follicle. Starting at the bottom part of the follicle, which is bulbous and contains the germinal matrix where cell division occurs, and the presumptive cortical cells, which form the bulk of a hair fiber, grow and elongate immediately above the bulb. Keratinization, the process of stabilization of the elongated cortical cells, occurs from the top of the bulb and is complete well before the fiber protrudes beyond the surface of the skin. The process of stabilization of the structure of the hair fiber involves the formation of disulfide bonds by oxidation of the thiol groups (—SH) present in the material immediately above the bulb and is almost absent in the keratinized fiber prior to its protrusion out of the follicle. That the molecular structure of the hair fiber is stabilized in the moist environment of the follicle means that the fiber is formed in mechanical equilibrium in the wet or near wet state. It follows that in the consideration of the physical properties of hair fibers in any arbitrary environment we must recognize that the fiber has shrunk from a water-swollen state at which mechanical equilibrium existed, and has not swollen from a dry state. As will be seen later in this discussion, this emphasis of the wet equilibrated state is necessary for our understanding of the variation of moisture uptake of different hair fibers. Water, by its plasticization of the biopolymeric structure of keratin, is a major contributor to the variation of the physical properties of these fibers. An understanding of the interaction of water in the keratin structure is vital. In basic terms we are examining the physical properties of the keratin-water system.
The polypeptide chains forming the keratin structure not only are cross-linked at intervals by covalent bonding formed by the cystine residues, but are held in various states of order by secondary bonds such as Van der Waals interactions, hydrogen bonds, and coulombic interactions sometimes referred to as “salt links” (5). These latter interactions arise from the presence of negatively charged ionised carboxylic acid groups (—COO−) and positive amino groups (—NH3+) formed on the side chains of acidic and basic residues. The presence of the latter bonds can be reduced or eliminated by placing the keratin fiber in an acidic aqueous medium (low pH) in which in the presence of excess hydrogen ions —COO− → — COOH; that is, the charge on the carboxylic acid groups is neutralized. A similar effect can be obtained in a basic aqueous medium (high pH) with the elimination of the charge on the amino groups (—NH3+ → —NH2) in the presence of excess hydroxyl ions. These latter interactions together with the other secondary bonds play an important role in the maintenance of the molecular and near molecular order present in α-ker-atin fibers. This order controls the freedom of movement and physical cooperation between the molecular chains forming the keratin, and obviously plays a major role in the mechanical and other physical properties of fibers. A discussion follows on a more detailed morphology of hair fibers indicating the relationships between the various components forming such fibers right down to the molecular level. The discussion is limited to those features of direct interest in the understanding of the properties of hair fibers. A detailed and wide-ranging discussion of morphology is left for others (6,7). There follows a discussion of the chemistry of the fibrous protein keratin of particular relevance to human hair and in particular the chemical factors that affect the physical behavior of the hair.
II. MORPHOLOGY
Fibrous α-keratins such as hairs, furs, and wools have in common the structural features of an external cuticle layer covering the cortex, the main material component of the fiber, with a central axial medulla present often in the coarser fibers. The cuticle consists of layers of scales each about 0.5 im thick in the case of human hair, and nearly 1 µm for wools. The number of scales present in the cross section of a fiber is also dependent on the type of fiber. In a newly formed human hair, up to 10 scales are present overlapping in one cross section, whereas in a wool fiber the scales are barely overlapping, resulting in mainly one scale thickness covering the fiber. The scale structure of the cuticle in the growing hair fiber in the follicle interlocks with the inner root sheath of the follicle. This inner root sheath travels outward at the same rate as the hair fiber and represents by its interlocking action the major mechanical stabilization of a hair fiber in the follicle. When a hair fiber is forcibly removed from the follicle, the inner root sheath is destroyed by this action. The inner root sheath as it moves outward is eliminated by chemical enzyme action, and the hair fiber is freed from its mechanical hold. The scales in all hairs form a ratchettlike structure resulting in a directional friction effect, which plays a vital role in the en...
Table of contents
- Cover
- Half Title
- Title Page
- Copyright Page
- Table of Contents
- About the Series
- Preface
- Contributors
- 1. Morphology and Properties of Hair
- 2. Physical Properties of Hair
- 3. Cleansing of Hair
- 4. Conditioning of Hair
- 5. Hair Styling/Fixative Products
- 6. Hair Waving
- 7. Hair Coloring
- 8. Fragrance for Hair Care Products
- 9. Ethnic Hair Care Products
- 10. Objective and Instrumental Methods for Evaluation of Hair Care Product Efficacy and Substantiation of Claims
- 11. Consumer Research Techniques
- 12. International Regulations for Hair Care Products
- Index
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Hair and Hair Care by Dale H. Johnson in PDF and/or ePUB format, as well as other popular books in Medicine & Chemistry. We have over one million books available in our catalogue for you to explore.