
- 176 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Chemistry of the f-Block Elements
About this book
Visual Spatial Enquiry explores visual and textual ways of working within spatial research. Architects and spatial thinkers from the arts, social sciences and humanities present rich case studies from remote and regional settings in Australia to the suburbs of Los Angeles, and from gallery and university settings to community collaborations in Mongolia. Through these case studies the authors reappraise and reconsider research approaches, methods and processes within and across their fields. In spatial research diagramming can be used as a method to synthesise complex concepts into a succinct picture, whereas metaphors can add the richness of lived experiences. Drawing on the editors' own architectural backgrounds, this volume is organised into three key themes: seeing, doing and making space. In seeing space chapters consider observational research enquiries where developing empathy for the context and topic is as important as gathering concrete data. Doing space explores generative opportunities that inform new and innovative propositions, and making space looks at ways to rethink and reshape spatial and relational settings. Through this volume Creagh and McGann invite readers to find their own understandings of the value and practices of neighbouring fields including planning, geography, ethnography, architecture and art. This exploration will be of value to researchers looking to develop their cross-disciplinary literacy, and to design practitioners looking to enhance and articulate their research skills.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
Topic
Ciencias físicasSubtopic
QuímicaChapter 1
INTRODUCTION
The lanthanide elements are usually defined as those in which the 4f-orbitals are progressively filled; this definition includes the elements Ce (cerium) to Lu (lutetium). Although not a lanthanide by this definition, La (lanthanum) is the prototype for the series and for practical purposes is usually included. The term ‘rare earth’ dates back to the early 19th century and is applied to elements 57 to 71 (La to Lu) and also to Y (yttrium), which is found in nature along with the lanthanides. Sc (scandium) is often also classed as a rare earth. The elements Th (thorium) to Lr (lawrencium) form the actinide series which is formally a 5f analogue of the lanthanide series, with Ac (actinium) as its prototype. This ‘actinide concept’, proposed in 1944 by Glenn Seaborg, solved the problem of where to fit the trans-uranium elements into the periodic table. The chemistry of the early actinides is quite distinct from that of their 4f congeners, and prior to 1944 the elements Th to U had been placed in the periodic table immediately below Hf, Ta and W. The late actinides show many similarities to the lanthanides.
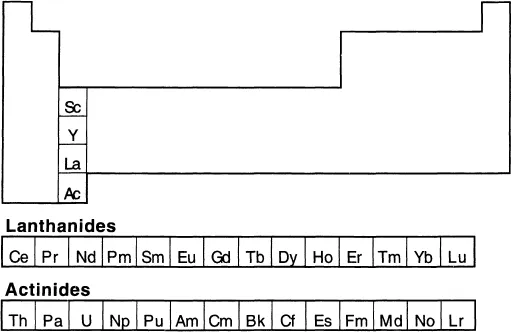
1.1 HISTORY
1.1.1 Lanthanides
The story of the lanthanides begins in 1787 when a young Swedish artillery officer, Lieutenant Carl Axel Arrhenius, who was a keen amateur geologist, was exploring a quarry at a small town called Ytterby, near Stockholm. He found a new, very dense black mineral which he named ytterbite’. At the time there was some speculation that the mineral might contain the recently discovered element tungsten, but the first serious chemical analysis was carried out in 1794 by Johan Gadolin, a Finnish chemist. Methods of chemical analysis were limited in the 18th century, but after a series of treatments with acids and alkalis, Gadolin was able to show that the new mineral contained oxides of iron, beryllium, and silicon and a new, previously unidentified ‘earth’ which he named ‘yttria’. (At the time, the term ‘earth’ was applied rather loosely to insoluble metal oxides.) Yttria was later shown to be a mixture of the oxides of six rare earth elements. A great deal of painstaking work was to follow during the 19th century: the first pure sample of dysprosium was obtained after 58 recrystallisations, and the first sample of Tm took 11000 crystallisations! With the discovery of lutetium (Lu) in 1907, the naturally occurring rare earths had all been isolated. Mosely’s pioneering work on X-ray spectroscopy in the early 20th century was invaluable in determining the purity (or otherwise) of newly discovered elements and also in pointing out the gaps in the Periodic Table. The missing element number 61, promethium, was synthesised and characterised in 1947, completing the lanthanide series. For an account of the history of promethium see Cotton (1999). Some of the history of the lanthanides is summarized in Table 1.1.
1.1.2 Actinides
Uranium was the first of the f-block elements to be discovered: its history dates back to 1789, the year of the French Revolution. Its story begins in Sankt Joachimstal in Bohemia, where silver had been mined since the 16th century. By the first half of the 17th century, silver mining had almost ceased, but bismuth and cobalt deposits were still being exploited, and a new shiny black mineral had been detected. This mineral was nicknamed ‘pitchblende’ from the German pech meaning ‘bad luck’ and blende meaning ‘mineral’. Pitchblende was first subjected to a chemical analysis by the German chemist Martin Klaproth who isolated what he called ‘a strange kind of half metal’ from the mineral. He named the new element ‘uranium’ after the recently discovered planet Uranus. Over the next century or so uranium deposits were found throughout the world: Cornwall in England, Morvan in France and in Austria and Romania. Uranium oxides and salts were widely used as pigments for ceramics and glass, and uranyl nitrate was also used to give a sepia tint to photographs. In the early part of the 20th century, the discovery of radium in pitchblende, and its medical applications, led to a further interest in seeking out deposits of this mineral. The largest use of uranium is now in nuclear power.
Thorium, the only other act...
Table of contents
- Cover Page
- Half Title
- Volume
- Title Page
- Copyright Page
- Dedication
- Contents
- Preface
- Chapter 1 Introduction
- Chapter 2 Spectroscopy
- Chapter 3 Coordination Chemistry
- Chapter 4 Organometallic Chemistry
- Chapter 5 Lanthanide Complexes as Catalysts and Reagents for Organic Reactions
- Index
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Chemistry of the f-Block Elements by Helen C. Aspinall in PDF and/or ePUB format, as well as other popular books in Ciencias físicas & Química. We have over one million books available in our catalogue for you to explore.