
eBook - ePub
Chemistry, Biological and Pharmacological Properties of Medicinal Plants from the Americas
- 240 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Chemistry, Biological and Pharmacological Properties of Medicinal Plants from the Americas
About this book
This volume is a compilation of plenary lectures presented at the IOCD/CYTED Symposium held in Panama City, Panama in 1997, and covers different aspects of research into plants from North, South and Central America. The topics treated all revolve around the chemistry, pharmacology, and biology of these plants. The importance of pharmaceuticals derived from plant sources is described, together with the potential of ethnomedicine for providing new leads in the search for bioactive constituents. The biodiversity of the Americas is underlined and an idea is given of the urgency with which the flora must be studied.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Chemistry, Biological and Pharmacological Properties of Medicinal Plants from the Americas by Kurt Hostettmann in PDF and/or ePUB format, as well as other popular books in Biological Sciences & Biology. We have over one million books available in our catalogue for you to explore.
Information
1. LESSONS FROM NATURAL MEDICINES
Department of Chemistry, Columbia University, New York, N.Y. 10027, USA
The chemistry dealing with natural products is the oldest branch of organic chemistry. Natural products chemistry started with the curiosity regarding odor, taste, color, folk medicinal cures, etc. In early days what is now known as natural products chemistry was focused on isolating the more readily available constituents and determining their structures. In certain cases this was followed by synthesis and elucidation of bio-synthetic routes. With the rapid advancement in isolation techniques and spectroscopy, structure determination became increasingly routine in most cases; the trend also shifted towards activity-monitored isolation and structural studies. However, in more recent years, it has become possible for organic chemists to direct their attention towards the mode of action, which almost invariably involves the interaction between a ligand and its biopolymeric receptor; a decade ago, such studies were impossible to be performed on a molecular structural level. Organic chemists, especially those involved in structural studies have the techniques, imagination, and knowledge with respect to the approach in such studies. However, nature is far more complex, and it is only with multidisciplinary collaborative research encompassing many disciplines that such targets can be successfully investigated. Why does sugar taste sweet or how do we perceive color? These are extremely challenging problems which at present cannot be answered even with a major multidisciplinary effort. We are at the starting point of a new field which is filled with exciting problems that have an immediate bearing directed towards a better understanding of Nature.
To understand the mode of action of folk herbs and related products from nature is even more complex because unfractionated or partly fractionated extracts are used. Nevertheless, the efficacy from this source has been proven over the years, in some cases covering thousands of years. The fact that natural medicines are attracting renewed attention is encouraging. Clarification of the constituents involved and understanding the mode of actions are clearly challenging but all the more worthwhile. In the following, some of our past studies in this area are outlined.
MICROBIAL PRODUCTS
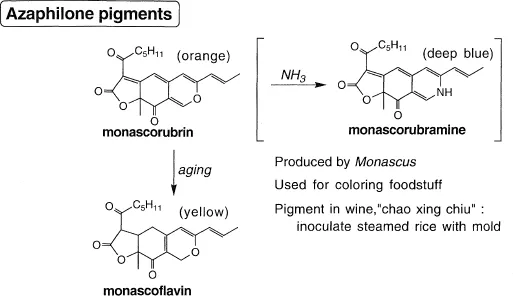
Monascombrin and Monascoflavin (Figure 1)
These orange and yellow pigments, respectively, isolated in 1926 by the late Eijiro Nishikawa (Tottori University) from Monascus purpureus Mentii, are widely used for coloring foodstuff in Asia and also are the main pigments of the popular Chinese wine “chao xing chiu”. M. Ohashi and others started working on these pigments collected earlier by Nishikawa. They belong to a group of pigments studied intensively by the Liverpool school of R. Robertson, B. Whalley and coworkers, and were generically called azaphilones because ammonia readily replaces the pyran oxygen with nitrogen yielding the violet nitrogen analog. The microorganism is spread on warm steamed rice to first produce the orange pigment; upon further fermentation, the pigment changes into the yellow monascoflavin, which gives rise to a higher quality wine. Besides structural studies, we performed our first biosynthetic studies to show the polyketide origin (Ohashi et al. 1960; Kumasaki et al. 1962; Kurono et al. 1963a).
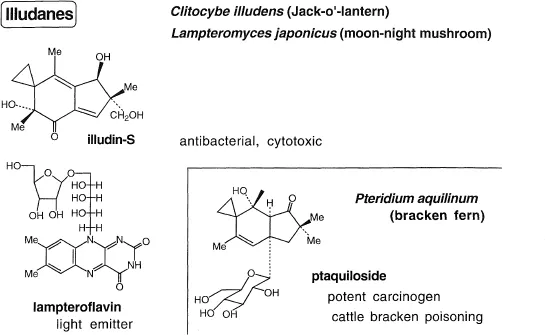
Illudin S (Figure 2)
Our studies on illudin-S (lampterol) started in Tokyo in 1962 and were completed in Sendai by Mamoru Ohashi, Masaru Tada and Yasuji Yamada (Nakanishi et al. 1965). It is a constituent of the bioluminescent and toxic “tsuki-yo-take” (Lampteromyces japonicus) that grows on rotten beech trees; the mushroom has been the cause of fatal accidents because of its similarity to edible mushrooms. Independent structural studies were carried out by Marjorie Anchel and Trevor McMorris at New York Botanical Garden (from Clitocybe illudens or Jack-o-lantern) (McMorris and Anchel 1963) and by Takeshi Matsumoto’s group at Hokkaido University (same source as ours) (Matsumoto et al. 1965). A large number of sesquiterpenoids with the illudan framework has since been identified. We were originally interested in the bioluminescent factor and toxin, but when an intensive screening test carried out by Takeda Company showed the mushroom to possess strong antitumor activity, extractions of the toxic and antitumor factors were carried out in parallel; the two turned out to be the same. We combined the collection of 300 kg of mushrooms with a fall group picnic. In groups of 2–3 we spread out into the fields of beech trees, collected the mushrooms in knapsacks, returned to the road and left them at the roadside to be picked up by a mini-truck; this was repeated several times until evening. The glow in the dark from the mushrooms dipped in methanol was an eery sight indeed. Although we attempted to isolate the bio-luminescent factor, I had to give up after cultivating a fluorescent fungi (Endo et al. 1970), because of my transfer to Columbia University; the factor was successful characterized in 1988 by Isobe and Goto (Isobe et al. 1988). The illudan skeleton attracted wide interest when a heat-unstable derivative of it was found to be responsible for the potent carcinogenecity of unboiled “warabi” (Hirono et al. 1984).
Chromomycins (Figure 3)
One of the earliest example of applications of exhaustive NMR decoupling coupled with extensive chemical studies established the structure of this group of antibiotics comprising a tricyclic skeleton and four new 2,6-deoxysugars. They were important clinical anticancer drugs; extensive studies are currently being performed at various institutes to understand their mode of interaction with DNA. The principal antibiotic chromomycin A3 was sold under the name of toyomycin; a number of similar compounds were isolated in the US, Russia, Italy and Japan but structures were unknown. A3 could be hydrolyzed with aqueous acetic acid to give four new deoxy sugars, the chromoses and the aglycone chromomycinone (CHR). Detailed 100 MHz NMR measurements, including double and triple resonance and solvent shift techniques to separate overlapping peaks, were carried out on the new sugars, the aglycone and derivatives. They were performed by Norman Bhacca (currently at Louisiana State University), a visiting scientist from Varian. The NMR studies carried out by Norman, some of the most extensive in those days, clarified all connectivities to disclose the full structure which to our surprise was found to contain one more carbon than originally thought.
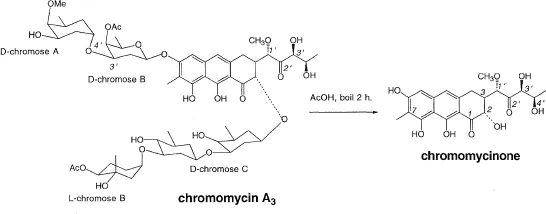
The original sugar connectivities in chromomycin A3 (Miyamoto et al. 1964, 1967) were revised later on the basis of extensive measurements by 300 MHz NMR (Thiem and Meyer 1979); in the original proposal the linkages at 3′ and 4′ of the D-chromose D moiety were interchanged and the stereochemistries at glycosidic centers were undetermined. The absolute stereochemistry was found (Harada et al. 1969) by the exciton chirality method in which the split circular dichroic curve resulting from coupling between the naphthalenoid transition and that of a benzoate chromophore at C-1′ was analysed. In the chromomycin studies, the Takeda team carried out beautiful and very extensive degradative studies while we were mainly involved in interpretations of spectral data. A chromomycin derivative has since been used as a template for application of red-shifted chromophores in the exciton chirality method (Cai et al. 1993).
Phytoalexins Produced by Sweet Potato (Figure 4)
Infection of plants by pathogens leads to the rapid production of antifungal compounds for self-defense. The molecular mechanism of this rapid and efficient defense is an important problem but is still obscure. When the sweet potato Ipomea batatas is invaded by the pathogenic black rot fungus Ceratocystis fimbriata and other fungi, ipomeamarone, a bitter sesquiterpenoid, rapidly accumulates in the infected tissue. The structure of this first phytoalexin was elucidated in 1956 by Kubota and Matsuura. As shown in Figure 4, inoculation of the roots with fungi or treatment with HgCl2, incubation for 3 days and extraction yielded ten new farnesol-derived ipomeamarones and...
Table of contents
- Cover Page
- Half Title
- Title Page
- Copyright Page
- Contents
- Preface
- List of Sponsors
- Contributors
- 1. Lessons from Natural Medicines—K. Nakanishi
- 2. Application of LC/MS and LC/NMR in the Search for New Bioactive Compounds from Plants of the Americas—K. Hostettmann and J.-L. Wolfender
- 3. The Quest for New Biologically Active Natural Products—R.P. Borris
- 4. Pharmacochemistry of New Compounds from South American Plants—R. Anton, A. Lobstein, M. Sauvain and B. Weniger
- 5. Bioassay Methods Useful for Activity-Guided Isolation of Natural Product Cancer Chemopreventive Agents—J.M. Pezzuto, L.L. Song, S.K. Lee, L.A. Shamon, E. Mata-Greenwood, M. Jang, H.-J. Jeong, E. Pisha, R.G. Mehta and A.D. Kinghorn
- 6. Biodiversity Conservation and Drug Development in Suriname—D.G.I. Kingston, A.A.E. Gunatilaka, B.-N. Zhou, M. Abdel-Kader, J. Berger, H. Van der Werff, R. Evans, L. Famolare, R. Mittermeier, S. Malone and J.H. Wisse
- 7. Antimicrobial Activities of Phytochemicals from British Columbian Medicinal Plants—G.H.N. Towers, A.K McCutcheon, H. Matsuura, J. Page, G. Saxena, S. Farmer, E. Gibbons, T.E. Roberts, L.A. Babiuk, L.M. Thorson, R.W. Stokes, P. Sokol and H. Klingemann
- 8. Bioactive Compounds from Panamanian Plants—M.P. Gupta, A. Marston and K. Hostettmann
- 9. Bioactive Natural Products of Medicinal and Agro chemical Interest from Selected Mexican Medicinal Plants—R. Mata, J.F. Rivero-Cruz and A. Rojas
- 10. The Brazilian Folk Medicine Program to Validate Medicinal Plants – A Topic in New Antihypertensive Drug Research—A.J. Lapa, M.T.R. de Lima-Landman, R.M. Cysneiros, A.C.R. Borges, C. Sonccar, I.P. Barett a and T.C.M. de Lima
- 11. Recent Developments in the Chemistry and Pharmacology of Boldo and Boldine—B.K. Casseis
- 12. Metabolic Engineering: A Strategy to Improve Plant Secondary Metabolite Production—R. Verpoorte, R. van der Heijden, J. Memelink and H.J.G. ten Hoopen
- Index