
Immunoassays
Development, Applications and Future Trends
- 438 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Immunoassays
Development, Applications and Future Trends
About this book
The concept behind this book is to provide a detailed and practical overview of the development and use of immunoassays in many different areas. Immunoassays are analytical tests that utilise antibodies to measure the amount, activity or identity of an analyte. This book is designed to provide a critical and helpful insight into the subject and to give the user practical information that may be of assistance in assay format selection, antibody generation/selection and choice of appropriate detection strategies. It is comprised of 13 chapters written by highly experienced researchers in the fields of antibody-based research, immunoassay development, assay validation, diagnostics and microfluidics.
Beginning with a comprehensive survey of antibodies, immunoassay formats and signalling systems, the book elucidates key topics related to the development of an ideal antibody-based sensor, focuses on the important topic of surface modification, explores key parameters in the immobilisation of antibodies onto solid surfaces, discusses the move to 'lab-on-a-chip'-based devices and investigates the key parameters necessary for their development. Three of the chapters are dedicated to the areas of clinical diagnostics, infectious disease monitoring and food security, where immunoassay-based applications have become highly valuable tools. The future of immunoassays, including next-generation immunoassays, electrochemical-immunoassays and 'lab-on-a-chip'-based systems, is also discussed. The book also covers the use of optical detection systems (with a focus on surface plasmon resonance) in immunoassays, provides a compilation of important, routinely used immunoassay protocols and addresses problems that may be encountered during assay development.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
Chapter 1
An Overview of Immunoassays
1.1 Introduction to Antibodies and Immunoassays
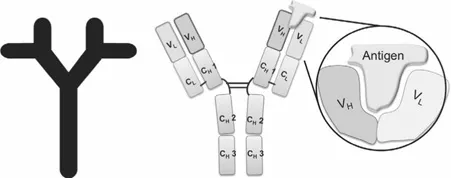
1.1.1 Polyclonal Antibody Production
Purification type | Methodologies involved in the purification |
Crude | Precipitation of a subset of total serum proteins that includes immunoglobulins. |
General | Affinity purification of certain antibody classes (e.g. IgG). |
Specific | Affinity purification of only those antibodies in a sample that bind to a particular antigen molecule. |
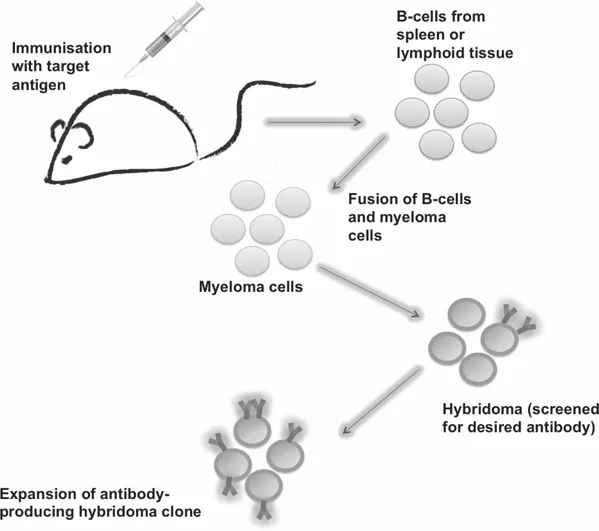
1.1.2 Production of Monoclonal Antibodies
1.1.3 Recombinant Antibody Fragments
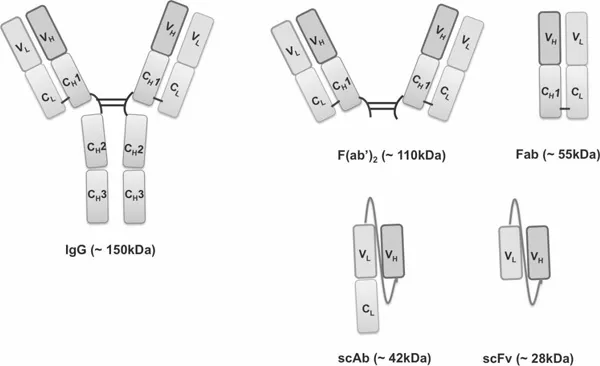
1.1.3.1 Production of recombinant antibodies by phage display technology
Table of contents
- Cover
- Half Title
- Title Page
- Copyright Page
- Contents
- Preface
- Glossary
- 1 An Overview of Immunoassays
- 2 Recombinant Antibodies for Diagnostic Applications: Design Considerations and Structural Correlates
- 3 Surfaces and Immobilisation Strategies for Use in Immunoassay Development
- 4 Immunoassay Validation
- 5 Lab-on-a-Chip Immunoassay Systems
- 6 Clinical Applications of Immunoassays
- 7 Immunoassay-Based Detection of Infectious and Parasitic Diseases
- 8 Detection of Food, Agricultural and Aquatic Contaminants
- 9 Next-Generation Immunoassays
- 10 Recent Trends and the Future of Electrochemical Immunoassay Systems
- 11 Optical Signal Transduction with an Emphasis on the Application of Surface Plasmon Resonance (SPR) in Antibody Characterisation
- 12 Protocols for Key Steps in the Development of an Immunoassay
- Index
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app