
- 658 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
This book is a detailed and comprehensive synthesis of the scientific study of aging. Dozens of contributions from leading scholars review various theories of aging, and molecular, cellular, biochemical and microbial aspects of aging, among just a few of the topics included. Authoritative, wide ranging and thorough, this book will act as a source for experimental design, a comprehensive description of age related diseases, and provide information of the latest molecular theories underlying their causes. Additionally, it will target industries involved in developing anti-aging drugs, post-graduate medical students, and university libraries.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
Section III
Diseases Associated with Aging and Treatment
10
The Premature Aging Characteristics of RecQ Helicases
Christ Ordookhanian, Taylor N. Dennis, and J. Jefferson P. Perry
Contents
Introduction
RecQ Helicase Domain Architecture
WRN RecQ Helicase
Werner Syndrome Clinical Phenotype
WRN Gene and Mutations
Overview of WRN Cellular Functions
WRN Exonuclease
WRN Multimerization
RQC and HRDC Domain Functions
WRN DNA Repair Functions
WRN Telomeric Maintenance Functions
WRN Roles in Supporting Heterochromatin
RECQL4 Helicase
RECQL4 Disease Syndromes
RecQ4 Biochemical Activities
RecQ4 DNA Replication Functions
RecQ4 Functions in DNA Repair
RecQ4 Functions in Telomere and Mitochondrial Maintenance
Conclusions
Acknowledgments
References
Introduction
The faithful replication of DNA and its maintenance and correct repair is critical to perturbing genetic changes that would otherwise drive increased aging, neurodegenerative disease, and cancer [1]. The RecQ helicase family of proteins function as key cellular mediators of genomic integrity, through their various roles in DNA metabolism, which includes functions in replication, recombination, and repair [2,3]. The RecQ helicase family is named after its founding member from E. coli [4], and RecQ proteins are distributed across the domains of life [5]. Where characterized, the RecQ helicases function through ATP hydrolysis to unwind and translocate along double-stranded (ds)DNA substrate in a 3′–5′ direction. This is in addition to having single-strand annealing properties. A number of alternate, structure-specific substrates have been defined for RecQ helicases, which contain similarities to intermediates of recombination and repair, providing further support for their functions in these DNA metabolism processes [6].
The human genome contains five RecQ helicases, RecQL1, BLM, WRN, RECQL4, and RecQL5, and their importance to human health is highlighted by mutations in WRN, BLM, and RECQL4 resulting in rare, distinct autosomal recessive disease syndromes [7]. So far, diseases caused by mutations in RecQL1 or RecQ5 have yet to be described [8]. The inherited RecQ disease syndrome share commonalities of increased cancer risk, dwarfism/a short stature, and have aging-related phenotypes to various degrees [9]. Hereditary mutations in BLM result in Bloom syndrome (BS) (OMIM #210900) that is unusual for a hereditary defect in having a broad-spectrum and marked cancer predisposition, with cancers that occur early and often in childhood [10]. Mutations in WRN give rise to the segmental progeroid disorder Werner syndrome (WS) (OMIM #277700), which has an early onset of aging that most closely resembles the normal aging process among the progerias [11–13]. Mutations in RecQ4 can give rise to three distinct syndromes with overlapping phenotypes that include skin abnormalities and skeletal defects [14]. The observed differences between the pathologies of these syndromes suggest that WRN, BLM, and RECQL4 have discrete functions within the cell.
WS is considered a prototypical progeroid syndrome [15], and is the focus of this chapter together with RecQ4 that also exhibits certain aging phenotypes. In terms of aging-related symptoms, WS and RecQ4 disease syndromes both have cataract formation and osteoporosis in common, but only WS has an observed predisposition to cardiovascular disease and diabetes mellitus. Also, the cancers observed between the WS and the RecQ4 spectra of disorders differ, and these also differ from BS. All cancer types occur in BS; sarcomas are common to WS patients; and osteosarcoma [16], squamous cell and basal cell carcinomas of the skin, and lymphoma are the most prevalent in individuals with RecQL4 mutations. BS-related studies could suggest some aging-related characteristics, such as hypogonadism and short stature, but the cancer clinical phenotype predominates. The broad spectrum of cancers observed in BS individuals includes leukemia, lymphomas, and carcinomas, there is the presence of synchronous or metachronous cancers, the average age of cancer diagnoses of a BLM cohort is approximately 26 years old, and the average life span for an individual with BS is 27 years. The driving force behind these cancers is elevated genome instability in BS cells, driven through sister chromatid exchanges (SCEs) and excessive crossovers between homologous chromosomes.
RecQ Helicase Domain Architecture
The central component of RecQ helicases is an architecture that belongs to the larger Superfamily 2 (SF2) helicases, with some features unique to RecQ proteins, consisting of helicase domains 1 and 2 (HD1, HD2) and a RecQ C-terminal (RQC) region (Figure 10.1). Within HD1 & 2 reside the classical seven-helicase sequence motifs (I, Ia, II, III, IV, V, and VI) that are known to couple ATP binding and hydrolysis with DNA unwinding. Mutation of these motifs can disrupt RecQ function [17] where a mutation within these motifs of WRN protein resulted in a Werner phenotype in mice tail-derived fibroblasts [18]. The Walker A and B Motifs (Motifs I and II) directly bind to ATP (or ATP analogs in the RecQ crystal structures) [19], as observed in the SF1 & 2 helicases, but there is also an extra region conserved in RecQs termed “Motif 0” that is a pocket that preferentially accommodates the adenine base of ATP. This pocket is similar to a pocket in RNA DEAD-box helicases known as the Q motif, suggesting a potential evolutionary link between these two classes of helicases. X-ray crystallography studies on several RecQ helicase structures, including human RecQ1 [20] and human BLM [21,22], have revealed that the RQC region is integral to the helicase core. This region is composed of a Zn2+-binding region and a winged-helix domain. The Zn2+ ion is chelated by four conserved cysteine residues, the mutation of which disrupts RecQ helicase function, and causes BS when mutated in BLM [23]. The winged-helix domain has high affinity for DNA likely providing substrate specificities, in addition to unique protein partner interactions for the RecQs.
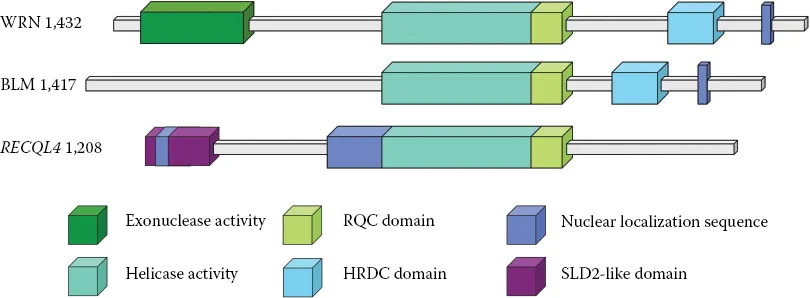
Figure 10.1Domain organization of three disease-related human RecQ helicases. All the RecQ homologous enzymes share a highly conserved helicase domain (teal). The nuclear localization sequence (blue) is present in the C-termini of WRN and BLM; RECQL4 has two NLSs which are both located in the N-terminus. The RQC domain (lime green) and the RNase domain C-terminal of the helicase (HRDC) (light blue) are located only in BLM and WRN following the helicase domain; an RQC domain has recently been identified in RECQL4 as well. Unique features include the N-terminal exonuclease domain (green) of WRN, and the N-terminal SLD2-like domain (purple) of RECQL4.
Regions outside of these conserved central helicase region are important for function, as their divergence between the RecQ family members likely provides distinct functionalities through differing DNA substrate specificities and protein partner in...
Table of contents
- Half Title
- Title Page
- Copyright Page
- Dedication
- Contents
- Preface
- One World, One Humanity
- Acknowledgments
- Editor
- Contributors
- Section I: Introduction to Aging
- Section II: Aging Hypothesis
- Section III: Diseases Associated with Aging and Treatment
- Section IV: Mechanisms of Aging
- Section V: Treatments in Aging
- Section VI: Healthy and Successful Aging
- Section VII: Anti-Aging Drugs
- Section VIII: Aging in Caenorhabditis elegans
- Section IX: Hibernation and Aging
- Section X: Mathematical Modeling of Aging
- Index
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Aging by Shamim I. Ahmad in PDF and/or ePUB format, as well as other popular books in Medicine & Geriatrics. We have over one million books available in our catalogue for you to explore.