
- 244 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Endometriosis in Clinical Practice
About this book
Endometriosis is on a worldwide increase and carries with it serious implications for women's reproductive health. Endometriosis in Clinical Practice brings together international experts to demonstrate what is known about the condition and the corresponding clinical implications for the patient suffering from it. Unlike many other books on this su
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Endometriosis in Clinical Practice by David Olive in PDF and/or ePUB format, as well as other popular books in Medicina & Ginecologia e ostetricia. We have over one million books available in our catalogue for you to explore.
Information
1.
Normal Cycling Endometrium: Molecular, Cellular, and Histologic Perspectives
Steven L.Young and Timothy S.Loy
CYCLIC ENDOMETRIAL CHANGES
The primary functions of the human endometrium are to allow the implantation of a normal embryo and provide mechanisms for the clearance of tissue and hemostasis at menstruation. At the same time, the endometrium must also provide a defense against invasion by potential pathogens and prevent the implantation of an abnormal embryo. In order to achieve these functions, the endometrium undergoes profound changes in structure and function during each cycle that result in defined periods of proliferation, embryo receptivity, and menstruation. The cyclic structural changes are evident on every level of examination, from gross inspection to electron microscopy. Both structural and functional changes are the result of changes in the molecular components of each cell, whereas a lack of appropriate cyclic changes is thought to underlie many common disorders, including abnormal uterine bleeding, infertility, endometriosis, and endometrial cancer. Therefore, a thorough understanding of the molecular and cellular alterations of the endometrium across the cycle should provide new approaches to the prevention, diagnosis, and treatment of endometrial disorders.
Considering that implantation of the embryo is fundamental to the survival of every human being at the earliest stage of his or her existence as an individual, it is remarkable that our current understanding of the molecular and cellular biology of the endometrium remains modest. Clearly, ethical and moral issues present significant hurdles to scientific inquiries into human embryo implantation, but an understanding of molecular and cell biology of the menstrual and immune functions of the endometrium also remains surprisingly incomplete. This chapter will provide an overview of the tissue, cellular, and molecular architecture of the endometrium, with an emphasis on changes across the cycle.
ENDOMETRIAL STRUCTURE—CELL TYPES
The endometrium is composed of multiple cell types, including epithelium, stroma, resident bone-marrow-derived immunocompetent cells, and blood vessel endothelium (Figure 1.1).
EPITHELIUM
The endometrial epithelium, embryonically derived from müllerian duct epithelium, forms a continuous layer, some of which is in direct contact with the uterine lumen
(luminal) and some of which lines thin, glandular invaginations of the lumen (glandular). The luminal epithelium consists of both ciliated and non-ciliated cells, whose relative number and morphology change over the cycle.
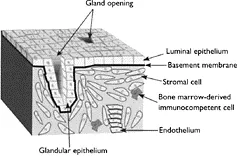
Figure 1.1 Structural organization of human endometrium.
The luminal epithelium is the first endometrial cell type to encounter an implanting embryo and the first cell type to encounter ascending foreign cells, including sperm and microbes. Thus, luminal epithelial cells must be immunologically unresponsive to foreign sperm, allow invasion by a normal embryo, and prevent invasion by abnormal embryos and foreign microbes. The luminal barrier to invasion is multifaceted and includes tight junctions between epithelial cells as well as specific apical membrane proteins and glycocalyx. During the embryo-receptive phase, the luminal epithelium undergoes a loss of apical-basal polarity, alterations in tight junction and cytoskeletal architecture, and thinning of the basal lamina. These structural changes are probably responsible, in part for increased trophoblast adhesion and the ability of trophoblasts to invade.1–4
Although the epithelial barrier to invasion is an important facet of endometrial defense, the barrier is probably weakened during implantation and certainly by menstruation. Also, the endometrium, like other mucosal surfaces, must detect potentially pathogenic microorganisms as early as possible to allow appropriate innate and adaptive responses. The immunologic problem of tolerating the presence of foreign sperm and invasion by a semi-foreign embryo while protecting against invasion by microbes and abnormal embryos was first recognized by the transplant immunologist Medawar over 50 years ago.5 To date, however, the mechanisms by which the endometrium accomplishes this immunologic feat remain an active subject of investigation.
The glandular epithelial cells, as the name implies, line a secretory gland, which during the secretory phase produces specific products thought to be important to the implanting trophoblast.6 These products include proteins and glycoproteins (e.g. prolactin and uteroglobin) as well as sialoglycoproteins (mucins). Changes in secretory glands are a major determining feature of endometrial differentiation, and many of the characteristic changes in the microscopic anatomy of the endometrium involve changes in the glands (see below).
STROMA
Endometrial stromal cells are derived from differentiated urogenital ridge mesenchymal cells immediately surrounding the müllerian duct, and the stroma is probably induced by the developing epithelium. The stroma cells are surrounded by a complex extracellular matrix7 produced, in large part, by the stromal cells themselves. Intermixed with the stromal cells are blood vessels and a variety of bone marrow-derived immunocompetent cells (see below). Perhaps the most distinctive change apparent in stromal structure across the cycle is decidualization. Decidualization involves enlargement and differentiation of the stromal cells, beginning with the perivascular stroma in the mid-secretory phase and continuing toward the stromal cells adjacent to the luminal and glandular epithelium. Decidualization is maintained during pregnancy.
BONE-MARROW DERIVED IMMUNOCOMPETENT CELLS
Lymphocytes (including natural killer (NK) cells) account for about 40% of the endometrial cells in early pregnancy, but little is known about their physiologic role. By far the most prevalent endometrial leukocyte in the secretory phase and early pregnancy is a specialized NK cell with granular morphology known as a large granular lymphocyte or uterine NK (uNK) cell. uNK cells have distinct differences from peripheral NK cells. uNK cells have cytolytic potential, which is low in the early proliferative phase but comparable to that of peripheral blood NK cells in the secretory phase and pregnancy.8 Furthermore, whereas 85–90% of peripheral NK cells display a CD16+ CD56dim immunophenotype, >90% of endometrial NK cells display a CD16- CD56bright immunophenotype.9,10 The proportion of CD16- CD56bright uNK cells rises from about 30–50% of total endometrial leukocytes in the proliferative phase to about 50–70% in the mid and late secretory phases, whereas CD16+ CD56dim NK cells remain at about 5–10% throughout the cycle.9,11 The recruitment of this rare subset of NK cells probably arises from expression of the CXCL12 chemokine by decidual cells and its receptor CXCR4 by peripheral CD16- NK cells.12
The other major type of leukocyte in the endometrium is the T lymphocyte. These cells make up approximately 5% of the total uterine cell population. Whether the numbers of T lymphocytes change during the cycle is unclear, although increased proliferation is seen in the secretory phase.13,14 Furthermore, the distribution and activity of T cells may undergo significant changes.9,11
Although macrophages and B-lymphocytes are found in the endometrium, they represent less than 10% and 5%, respectively, of endometrial leukocytes. Interestingly, lymphoid aggregates containing a B-cell core surrounded by CD8+ T cells and a halo of macrophages have been observed in the endometrium, and the size of these aggregates increases in the secretory phase.11,14,15 In addition, prominent infiltrates of neutrophils are seen on histologic sections taken from the very late secretory phase.
ENDOTHELIUM
Radial arteries penetrate the myometrium and split into basal and spiral arteries. The basal arteries form a rich network of anastomoses, supplying the relatively stable endometrial basalis and, like the basalis, undergo little cyclic change. In contrast, the
spiral arteries do not anastomose and change markedly over the cycle, to supply the first proliferating and then differentiating endometrial functionalis.
ENDOMETRIAL STRUCTURE: HISTOLOGY
It has been almost 100 years since Hitschmann and Adler first reported cyclic changes in the microscopic architecture of endometrial functionalis, and more than 50 years since Noyes et al. established the basic histologic criteria currently used by pathologists for the assessment of endometrial differentiation on endometrial biopsies stained with hematoxylin and eosin.16–18 A careful reassessment of the classic histologic dating criteria using fertile subjects, modern cyclemonitoring techniques, and modified analytic methods has confirmed that the histologic changes described in 1950 represent a good description of the usual cyclic changes in endometrial structure and composition, but has also suggested that classic histologic evaluation of the endometrium is insufficiently precise to be used for clinical evaluation of endometrial function.19
Most authors describe cyclic changes in endometrial histology using an idealized 28- day cycle, with menses on day 1, lutenizing hormone (LH) surge on day 13 (d13), ovulation on d14, followed by 14 more days of secretory phase. Typical cyclic changes in endometrial morphology, demonstrated in Figure 1.2, have been extensively described and will only be outlined here.17,18,20,21
The proliferative phase follows the menses and thus is initially characterized by re-epithelialization, which begins as early as d2 of the cycle.22 By the early proliferative phase (d5–7; Figure 1.2A), the endometrial epithelium covers the surface, and straight glands with a small circular crosssection are evident. The luminal and glandular epithelial cells are short, with basal nuclei, whereas the stroma is composed of oval cells with little cytoplasm. In early proliferative endometrium, mitotic figures are rare in both epithelium and stroma. Under the influence of increasing levels of estrogen, the midproliferative endometrium, (d8–10; Figure 1.2B) is characterized by increased epithelial and stromal mitosis, more columnar epithelium with pseudostratified nuclei, slightly coiled glands, and stromal edema. Estrogen continues to rise markedly in the late proliferative phase (d11–14; Figure 1.2C), causing continued glandular mitoses and increased stromal mitoses. The glands become more tightly coiled, with a wide lumen, and the glandular epithelium shows maximal pseudostratification, whereas the stromal edema lessens.
The high, sustained levels of estradiol trigger the LH surge, which in turn triggers ovulation about 34–36 hours after the onset of the s...
Table of contents
- COVER PAGE
- TITLE PAGE
- COPYRIGHT PAGE
- LIST OF CONTRIBUTORS
- PREFACE
- 1: NORMAL CYCLING ENDOMETRIUM: MOLECULAR, CELLULAR, AND HISTOLOGIC PERSPECTIVES
- 2: ENDOMETRIOSIS AND IMPLANTATION
- 3: THE ENDOMETRIUM AND ANGIOGENESIS
- 4: EPIDEMIOLOGY OF ENDOMETRIOSIS
- 5: PATHOGENESIS OF ENDOMETRIOSIS
- 6: ENDOMETRIOSIS IS AN INFLAMMATORY DISEASE
- 7: CYTOKINE REGULATION
- 8: MATRIX METALLOPROTEINASES AND ENDOMETRIOSIS
- 9: THE BIOLOGY OF ENDOMETRIOSIS: AROMATASE AND ENDOMETRIOSIS
- 10: ANIMAL MODELS
- 11: SYMPTOMS
- 12: DIAGNOSIS
- 13: ASSESSING HEALTH STATUS IN ENDOMETRIOSIS
- 14: MEDICAL THERAPY OF ENDOMETRIOSIS
- 15: SURGICAL TREATMENT OF WOMEN WITH ENDOMETRIOSIS
- 16: ASSISTED REPRODUCTION AND ENDOMETRIOSIS
- 17: THE STRUCTURE AND FUTURE OF ENDOMETRIOSIS RESEARCH