1.1 Introduction
It was pointed out in the preface that clinical trial sponsors are actively looking for ways to develop more efficient approaches to designing, conducting and analyzing trials. This can be accomplished most effectively through a comprehensive quantitative assessment of available options, including innovative trial designs and statistical methods. Clinical Scenario Evaluation (CSE) was introduced in Benda et al. (2010), and subsequently refined in Friede et al. (2010) and other publications, as an efficient quantitative approach to evaluating trial designs and data analysis methods in individual clinical trials or, more generally, in clinical development programs.
A general CSE-based approach to designing a clinical trial incorporates a thorough assessment of multiple competing strategies which enables the trial’s team to assess the pros and cons of the applicable trial designs and analysis techniques and to examine their sensitivity to potential changes in the underlying assumptions. This approach has the potential to help trial sponsors set up more robust designs that avoid overly optimistic assumptions that are still very common in Phase III trials (Gan et al., 2012) and, along the same line, improve on simplistic or suboptimal analytical methods such as inefficient multiplicity adjustments that are often employed in clinical trials; see, for example, PREVAIL trial (Beer et al., 2014) or APEX trial (Cohen et al., 2013; Cohen et al., 2014). In fact, CSE has been successfully leveraged in multiple Phase II and III clinical trials and led to tangible improvements compared to ad-hoc approaches to trial design and analysis. Examples and references can be found in Benda et al. (2010), Friede et al. (2010), as well as more recent publications, including Dmitrienko, Paux and Brechenmacher (2015) and Dmitrienko et al. (2016).
As a powerful approach to improving the probability of success and building robust trial designs and analysis strategies, the general CSE framework will play a central role in this book. The guiding principles of CSE will be introduced in Section 1.2 and will be applied in Chapters 2, 3 and 4. The discussion of CSE applications in these chapters will focus mostly on efficacy assessments in a single clinical trial. However, it is important to note that many other applications fall within the CSE domain. This includes, for example, a systematic assessment of safety signals as well as CSE-based evaluations at the program level. For a discussion of key considerations related to applying CSE techniques to multiple clinical trials within the same development program, see Benda et al. (2010).
It is important to explore the connections between CSE and clinical trial optimization. As pointed out in Benda et al. (2010), due to its emphasis on identifying best-performing designs and analysis strategies in clinical trials, CSE provides a foundation for developing a holistic approach to optimizing drug development. The key principles of CSE-based clinical trial optimization will be introduced in Section 1.3 and illustrated throughout this book. Practical and easy-to-implement clinical trial optimization strategies will be emphasized in the book. The resulting strategies provide quantitative information to support internal decision making and efficient dialog with external clinical experts and regulators. To facilitate the application of CSE and optimization methods to real-life trials, multiple case studies and R code will be provided in Chapters 2, 3 and 4.
1.2 Clinical Scenario Evaluation
This section introduces the key principles and building blocks of the general CSE approach with emphasis on the trial-level and analysis-level evaluations introduced in Benda et al. (2010). The process of setting up a CSE framework and defining its components in real clinical trials will be illustrated in Section 1.2.2 using an R package that was designed to support CSE in clinical trial applications (Mediana package).
1.2.1 Components of Clinical Scenario Evaluation
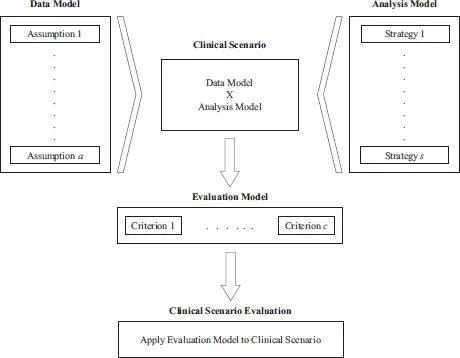
It is broadly recognized that evaluation of candidate trial designs and analysis methods in clinical trials is, by nature, a multidimensional process. Benda et al. (2010) proposed to decompose this complex problem into a small number of components that define the core building blocks of the overall CSE framework. These components were referred to as assumptions, options and metrics in the original CSE publications. Dmitrienko et al. (2016) revised this terminology to introduce more descriptive labels for each CSE component:
• Data models, previously known as assumptions, define the process of generating the trial data.
• Analysis models, previously known as options, specify the analysis strategies applied to the trial data.
• Evaluation models, previously known as metrics, specify the criteria for evaluating the performance of the analysis strategies applied to the trial data.
Within the data and analysis models, multiple statistical assumptions or analysis strategies can be specified and the combination of these two models represents a clinical scenario. CSE focuses on the application of the criteria defined in the evaluation model to the clinical scenarios of interest. A visual representation of the CSE framework is provided in Figure 1.1.
This decomposition into three independent components provides a structured framework for clinical trial simulations which enables clinical trial researchers to carry out a systematic quantitative assessment of the operating characteristics of candidate designs and statistical methods to characterize their performance in multiple settings. Ultimately, this assessment supports the selection of a robust approach to clinical trial design and analysis which demonstrates optimal performance.
It was emphasized in the introduction that, within the CSE framework, emphasis is placed on realistic assumptions as opposed to simplistic assumptions that may be introduced to streamline the process of designing a clinical trial or simplify the data analysis models. For example, when designing a trial with a time-to-event endpoint, it is quite common to assume that this endpoint follows an exponential distribution because this distribution supports the assumption of proportional hazards and leads to a straightforward closed-form expression for the required number of events in the trial. CSE encourages clinical trial sponsors...