
- 672 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Antioxidant Status, Diet, Nutrition, and Health
About this book
This is the first book to integrate the biological, nutritional, and health aspects of antioxidant status. Fifty contributors integrate and transfer the knowledge of free radicals and antioxidants from the test tube to the laboratory of the biologist, clinical nutritionist, and medical researcher, as well as to the office of the dietician, nutritionist, and physician. Topics examined include factors affecting and methods for evaluating antioxidant status in humans; effect of diet and physiological stage (infancy, aging, exercise, alcoholism, HIV infection, etc.) on antioxidant status; and the role of antioxidant status in nutrition, health, and disease.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Antioxidant Status, Diet, Nutrition, and Health by Andreas M. Papas in PDF and/or ePUB format, as well as other popular books in Medicine & Nutrition, Dietics & Bariatrics. We have over one million books available in our catalogue for you to explore.
Information
Part 1
Antioxidant Status in Humans
• Chemistry of Active Oxygen Species and Antioxidants
• Determinants of Antioxidant Status
• Evaluation of Antioxidant Status
1
Chemistry of Active Oxygen Species and Antioxidants
Research Center for Advanced Science and Technology
University of Tokyo, JAPAN
University of Tokyo, JAPAN
FREE RADICALS AND ACTIVE OXYGEN SPECIES
Introduction
Free radicals are chemical species, which have unpaired electrons. Molecules are composed of atoms and electrons. Electrons are present generally in pairs. However, under certain conditions, molecules have unpaired electrons and as such they are called free radicals. Unpaired electrons usually seek other electrons to become paired. Thus, free radicals are in general reactive and attack other molecules, although some radicals are not reactive but stable enough to have long life. Examples of reactive free radicals are the hydroxyl (HO•) and alkoxyl (LO•) radicals, while the nitric oxide (•NO), vitamin E (to-copheroxyl), and vitamin C (dehydroascorbate) radicals are examples of stable radicals.
Active oxygen species (also known as reactive oxygen species) denote oxygen-containing molecules, which are more active than the triplet oxygen molecule present in air. Superoxide (O2•−), hydrogen peroxide (H2O2), hydroxyl radical, and singlet oxygen (1O2) are accepted as typical active oxygen species, but in broader sense, other species such as alkoxyl radical, peroxyl radical (LO2•), nitrogen dioxide (NO2•), lipid hydroperoxide (LOOH), protein hydroperoxide, and hypochlorite (HOCI) are also considered as active oxygen species. Some of them have unpaired electrons and are free radicals, but others are not. Table 1 summarizes the active oxygen species, which are relevant to lipid peroxidation and oxidative stress in vivo. Nitric oxide and thiyl radical (RS•), which do not bear unpaired electrons on oxygen are also included.
Radicals | Non-radicals | ||
|---|---|---|---|
O2•− | superoxide | H2O2 | hydrogen peroxide |
HO• | hydroxyl radical | 1O2 | singlet oxygen |
HO2• | hydroperoxyl radical | LOOH | lipid hydroperoxide |
L• | lipid radical | Fe=O | iron-oxygen complexes |
LO2− | lipid peroxyl radical | HOC1 | hypochlorite |
LO− | lipid alkoxyl radical | ||
NO2• | nitrogen dioxide | ||
•NO | nitric oxide | ||
RS• | thiyl radical | ||
P• | protein radical | ||
Physiological Functions and Effects
Active oxygen and related species play an important physiological role and, at the same time, they may exert toxic effects as well. The active oxygen species are essential for production of energy, synthesis of biologically essential compounds, and phagocytosis, a critical process of our immune system. They also play a vital role in signal transduction, which is important for cell communication and function. On the other hand, there is now increasing evidence which shows that these active oxygen species may play a causative role in a variety of diseases including heart disease and cancer, and aging. Consequently, the role of antioxidants, which suppress such oxidative damage, has received increased attention. It is important to elucidate the mechanisms and dynamics of the oxidative damage in order to understand its biological significance and develop strategies to prevent it. Both active oxygen species and antioxidants are double-edged swords and the balance of their beneficial and toxic effects is determined by the relative importance of many competing biological reactions.
Formation of Free Radicals and Active Oxygen Species
Free radicals and active oxygen species are formed by various extrinsic and intrinsic sources such as light, heat, and metals. They are formed in vivo by various ways at different times and sites as summarized in Table 2.
Reactions of Free Radicals and Active Oxygen Species and Their Reactivities
Free radicals and active oxygen species attack lipids, sugars, proteins, and DNA and induce their oxidation, which may result in oxidative damage such as deterioration of foods, membrane dysfunction, protein modification, enzyme inactivation, and break of DNA strands and modification of its bases. The important reactions of free radicals underlying these events may be classified into the following categories:
1. Hydrogen Atom Transfer Reaction
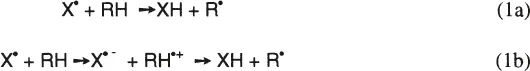
2. Addition Reaction
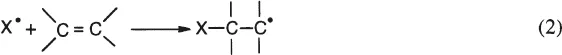
3. Aromatic Substitution Reaction

4. β-Scission Reaction
5. Coupling Reactions

The hydrogen atom transfer reaction is an important key step in lipid peroxidation, protein modification, and DNA damage. It may proceed by direct hydrogen atom abstraction by free radical (reaction la) or by electron transfer reaction followed by proton transfer (reaction lb). The hydrogen atom abstraction from lipids by peroxyl radicals is the key step in determining the rate and product distribution in lipid peroxidation. They are determined by the reactivities of the attacking radicals as well as those of lipids. Table 3 shows the rate constants for hydrogen atom abstraction from lipids by various free radicals. The reactivities of free radicals toward lipids vary quite extensively by a factor of 1010! The reactivity of the free radical X• can be estimated by the bond dissociation energy of the X-H bond, BDE(X-H). The larger the bond dissociation energy of the X-H bond, the higher the reactivity of X• radical. The bond dissociation energy of HO-H bond is 119 kcal/mol (498 kJ/mol), while that of LOO-H is 88 kcal/mol (368 kJ/mol), and the HO• radical is much more reactive than peroxyl radical in hydrogen atom abstraction.
The bond dissociation energy of the C-H bond being attacked is also important in determining the rate. The bond dissociation energies of the primary, secondary, and tertiary C-H bonds of saturated hydrocarbons are 98, 95, and 92 kcal/mol and those of secondary allylic and bisallylic C-H bonds are 85 and 75 kcal/mol, respectively (Figure 1). The weaker the C-H bond, the faster the hydrogen is abstracted. Hydroxyl radical is so reactive that it is capable of abstracting any type of hydrogen from lipids rapidly, whereas peroxyl radical is much less reactive and it selectively abstracts only reactive hydrogen such as bisallylic hydrogen of polyunsaturated lipids.
Active oxygen species | Formation |
|---|---|
Superoxide (Hydoperoxyl radical) O2•− (HO2•) | Enzymatic and non-enzymatic one electron reduction of oxygen |
Hydroxyl radical, HO• | Radiolysis of water, metal-catalyzed decomposition of hydrogen peroxide, interaction of NO and superoxide |
Alkoxyl and peroxyl radicals LO•, LO2• | Metal-catalyzed decomposition of hydroperoxides |
Hydrogen peroxide, H2O2 | Dismutation of superoxide, oxidation of sugars |
Iron-oxygen complex, Fe=0, etc | Hemoglobin, myoglobin, etc. |
Singlet oxygen, 1O2 | Photosensitized oxidation, bimolecular interactions between peroxyl radicals, reaction of hypochlorite and hydrogen peroxide |
Lipid and protein hydroperoxides | Oxidation of lipids and proteins |
Nitrogen dioxide, NO2• | Reaction of peroxyl radical and NO, polluted air and smoking |
Nitric oxide, •NO | Nitric oxide synthase, nitroso thiol, and polluted air |
Thiyl radical, RS• | Hydrogen atom tr... |
Table of contents
- Cover
- Title Page
- Copyright Page
- Preface
- Foreword
- Contributors
- Dedication
- Introduction
- Table of Contents
- Part 1. Antioxidant Status in Humans
- Part 2. Effect of Diet on Antioxidant Status
- Part 3. Major Dietary and Other Antioxidants
- Part 4. Physiological Stage and Antioxidant Status
- Part 5. Proposed Modes of Action of Antioxidants
- Part 6. Antioxidants and Disease
- Part 7. Current Issues and Emerging Research
- Epilogue
- Index