![]()
1 Introduction to Biomedical Engineering Design
This text is designed to cover the design of biomedical engineering devices and/or systems. It is intended as a reference to guide your and your classmates’ thoughts and actions in design. It is based upon the authors’ experiences in industry with the practice of engineering design and the authors’ experiences and those of their students (both undergraduate and graduate) in the learning of design and the initiation of design projects from a multitude of sources. Also covered will be the topics of relevance from the authors’ experiences in court with device and process failures. With your prior instruction in engineering, with your projects of interest, it is anticipated that this text will be of value to you in the initiation and potential completion of a design project relevant to this field.
What is relevant to this field? Biomedical engineering can be very broad in scope, dependent on interests and circumstances. Biomedical engineers are expected to have some familiarity with medical devices, their design, their regulation, and use. They are further expected to consider safety aspects of the devices and should consider the potential misuse of a device. Designers may be expected to involve themselves in the improvement of a process, such as the processing of patients in a hypertension or cancer clinic. They may get involved in biotechnology to manufacture products derived from mammalian cells; they may wind up in the manufacturing of implant devices for the treatment of diabetes. They may design a specialized brace for a single individual or design a medical device to be used by thousands of patients. It is vital to understand the many meanings of the term design and have some experience at problem-solving using the design principles and other considerations to be outlined in this text.
1.1 WHAT IS DESIGN?
It is useful to discuss design from two viewpoints this early in the text, first by discussing what it is not, then by discussing what it is and the many forms of it.
Design is not research, which may be defined as “a careful investigation or study, especially of a scholarly or scientific nature.”1 A design task may require research to accomplish a task, but it typically involves the integration of knowledge, rather than the generation of knowledge. Research may be done into the process of design and as such is sponsored by such groups as the National Science Foundation (see www.eng.nsf.gov/dmii/index.htm for the design, manufacturing, and industrial innovation research division). A researcher (scientist) may study nuclear fusion, but an engineer (designer) must be involved in the construction of a nuclear power plant.
On the other hand, design is not craftsmanship. Designers are not, nor should they be viewed as, craftsmen. This work will involve brains and skills, not just skills.
Design as an action verb is
Design work thus does not necessarily involve the manufacture of a physical device; it can be a plan or process, or a study to determine the same. Naturally, it can range from this level to the complete specification of a device and its manufacture.
Design as a noun (thing) incorporates the following:
A drawing or sketch; especially a detailed plan for construction or manufacture
The purposeful arrangement of parts or details
The art or practice of making designs
An ornamental pattern
A plan or project
A reasoned purpose; intent
Often a secretive plot or scheme (Latin)1
Each of these terms has validity in the types of work that will be discussed in this text, especially if the device is useful and novel, and therefore potentially patentable. Even the ornamental pattern qualifies as it is a product of the intellect, is therefore an invention, and may qualify as patentable intellectual property. How does a secretive plot qualify? Perhaps under the category of “trade secret,” for example, as is the recipe for the manufacture of Coca-Cola.
1.2 WHAT IS THE THRUST OF THIS TEXT?
This text is aimed at introducing one to the application of design processes to a wide category of design problems in biomedical engineering. It is anticipated that the user of this text will be involved during the reading of this text in one or more design projects and/or exercises. It likely will best be used in parallel with some early design exercises, and then referred to occasionally as a major design project is pursued. It is meant to be a part of the learning triad of hear/see/do, but not all of it.
The text also attempts to place the various steps in the design process in a logical order, typically that followed in engineering best practices for conducting and completing a design project. The process is generic and flexible, so that processes may be included or not, depending on the project.
1.3 WHAT MIGHT BE DESIGNED?
A partial listing of senior-level (and some graduate-level) design projects follows:
Biomedical devices
Modified patient brace for an individual
Patient (e.g., Alzheimer’s) (or pet) tracking device
Development of a hand exerciser
Improved safety warning system for an intensive care unit
Improved patient monitoring for premature infants
Development of a voice training system for patients with Parkinson’s disease
Development of a surgical tool for use in spina bifida surgery
Development of an adjustable tray for a spinal cord-injured patient
Modification of a riding mower for use by a paraplegic
Development of a laser spot size measurement system for use in throat surgery
Biomedical systems
Improved patient record-keeping system for a perioperative surgical unit
Revised and improved vaccine database system
Comprehensive pain clinic data collection/billing system
Development of a prostate cancer screening test
Development of a skin disease database
Development of a research ward database system
Improved feeding apparatus for cystic fibrosis patients
Development of a device for laparoscopic band pressure regulation
Biofeedback system for wheelchair propulsion systems
Development of a cauterizing biopsy catheter
Development of a drug-eluting stent
Biomedical processes
A study of patient flow in an emergency room
Improved patient communication in a breast cancer clinic
Determination of clinic space and facility needs
Development of a system to measure foot impressions and transmit same
Optimization of T-cell trapping in a microfluidic device
Note the key words improve, develop, revise, or study. Note also the key words device, process, and system. Each of these terms will see major elaboration in the ensuing chapters. Design will on occasion involve invention but generally will involve an application (extension) of existing technology. In addition, as will be noted in the solution of design problems, there will be no “exact answer,” but instead, there will be best attempts given constraints involving timing and financing of project work.
1.4 THE ESSENTIALS OF DESIGN—OVERVIEW
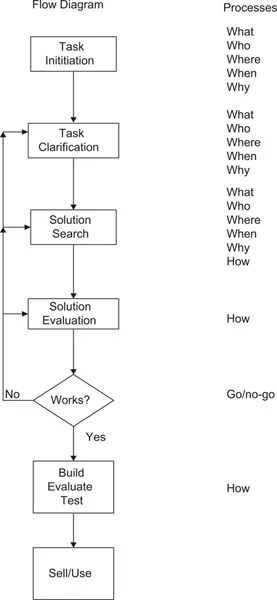
A well-written newspaper article quickly answers the following questions: Who? What? Where? When? Why? How? The process of design typically involves such considerations, with the addition of decision points in the design process as outlined in Figure 1.1. The generic design process, the flowchart on the left-hand side, outlines the process from design task initiation (from whatever source) to task completion. The center section outlines the process of task clarification, the search for solutions and evaluations of the same, and the generally necessary reiterations of the same until an acceptable solution is reached.
FIGURE 1.1A generalized flowchart for the design process.
On the right-hand side are listed the who/what/why/when/where/how questions normally asked at each phase, with the addition of the go/no decision part. The most critical part of this listing is the initial “what?” elucidation—if the design task is not adequately described at the outset, the entire process from then on is generally wasted time and money! If one properly defines the problem (i.e., understands the who/what/why/when/where part), then one can hope for a tracing directly to the solution evaluation section. If the solution is wrong, or if the problem definition is wrong, one will have to backtrack and rework the overall solution. The most vital part of the design work will be to figure out what it is that one is asked to do.
Part of this problem will hopefully be minimized by your (and/or your team’s) prior educational experiences, which should have included medical nomenclature, some systems physiology, and medical instrumentation. If one is working with a non-engineer on your design project, some new communication skills may be needed. This will be especially true in dealing with most physician collaborators, who commonly go from diagnosis to treatment (or therapy) on a generally non-modifiable patient, while the designer is charged with the modification of a device or process.
1.5 BIOMEDICAL ENGINEERING DESIGN IN AN INDUSTRIAL CONTEXT
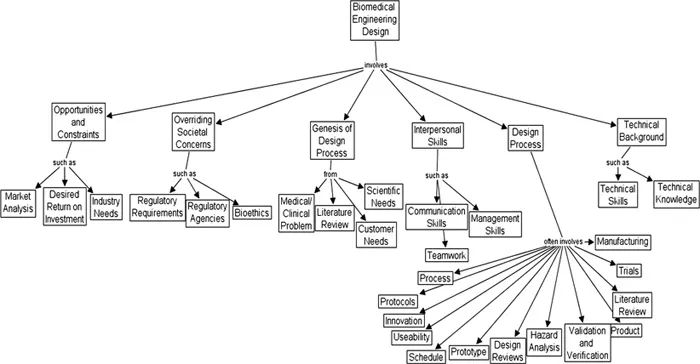
Figure 1.2 is a concept map2 describing, in a hierarchical fashion, the overall elements of the biomedical engineering design process in the context of society. It is a consensus document as to the generic elements that must be considered in the overall design process. To understand this system, one normally would read from top to bottom and from left to right.
FIGURE 1.2The generic biomedical engineering design process.
Biomedical engineering design projects generally involve opportunities, subject to certain constraints. A company will not typically pursue a project unless a market analysis has been done, there exists a potentially desired return on investment, and/or the product is a fit with industry needs (and intellectual property rights may be retained). [In contrast, for a student design process in a generic academic setting, projects are generally proposed to students based upon the potential advisors’ (medical faculty, engineering faculty, industry advisor) needs, tempered by what might be expected from a student design team, etc.]
For industry, there are several overriding societal concerns involved in the design of devices and products; these involve multiple regulatory requirements (licensing, waste disposal, liability issues, etc.), regulatory agencies (the Food and Drug Administration (FDA), etc.), and bioethical constraints (animal care rules, human subjects committee approvals, etc.). [For student projects, human subjects committee approvals are sometimes necessary, a knowledge of FDA rules is useful, a project may brush with the group “Persons for the Ethical Treatment of Animals” (PETA), etc.]
Design projects typically arise from studies of medical and clinical problems (such as device complaints), a literature review, the scientific project needs of clinical and academic investigators, and occasionally the needs of individual customers.
Design projects seldom involve a single designer, and thus, issues of interpersonal skills, such as communication skills, management skills (especially time management), and teamwork skills, come to the forefront of the design process.
The design process itself may involve developing a process (as opposed...