
- 192 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
This is a new undergraduate textbook on physical chemistry by Horia Metiu published as four separate paperback volumes. These four volumes on physical chemistry combine a clear and thorough presentation of the theoretical and mathematical aspects of the subject with examples and applications drawn from current industrial and academic research. By using the computer to solve problems that include actual experimental data, the author is able to cover the subject matter at a practical level. The books closely integrate the theoretical chemistry being taught with industrial and laboratory practice. This approach enables the student to compare theoretical projections with experimental results, thereby providing a realistic grounding for future practicing chemists and engineers. Each volume of Physical Chemistry includes Mathematica® and Mathcad® Workbooks on downloadable resources.
Metiu's four separate volumes-Thermodynamics, Statistical Mechanics, Kinetics, and Quantum Mechanics-offer built-in flexibility by allowing the subject to be covered in any order.
These textbooks can be used to teach physical chemistry without a computer, but the experience is enriched substantially for those students who do learn how to read and write Mathematica® or Mathcad® programs. A TI-89 scientific calculator can be used to solve most of the exercises and problems.
® Mathematica is a registered trademark of Wolfram Research, Inc.
® Mathcad is a registered trademark of Mathsoft Engineering & Education, Inc.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Physical Chemistry by Horia Metiu in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Chemistry. We have over one million books available in our catalogue for you to explore.
Information
1
GENERALITIES ABOUT THE RATES OF CHEMICAL REACTIONS

Introduction
§1. Chemical Kinetics: What is it? To explain what chemical kinetics does, I use the reaction
(1.1) |
as an example. A mixture of N2 with H2 held at high temperature and pressure, in the presence of the right catalyst, produces ammonia. After mixing the reactants, the number of moles of ammonia increases in time and those of nitrogen and hydrogen decrease. Chemical kinetics studies how fast the amounts of reactant and product change during a reaction.
§2. What is it Good For?
No chemical plant is designed without a thorough kinetic study of the main reactions taking place in it. The reaction rates control the productivity, the cost of the product, and the profit of the plant.
Imagine that you are designing a plant in which the reaction A ⇌ B is performed. You know that if you wait long enough the reaction reaches equilibrium. When that happens you get the highest possible yield of B. You might consider keeping the reaction going for the time te that is needed for reaching equilibrium, and get the best yield. This seems reasonable: why not go for the best? However, time is money and getting the best yield is not necessarily the best plant management. If I have to wait for hours for the reaction to reach equilibrium, the productivity is low. A factory that has fixed costs (salaries, amortization on investment, etc.) cannot afford to produce a small amount of “stuff” per hour, unless the “stuff” can fetch a very high price. I could make the reactor larger and get a large amount of B per batch. But, large reactors are more expensive and this initial cost will drive up the cost of what I am making.
After pondering all these constraints, you may start to wonder: what will happen if I don’t wait until equilibrium is reached? This is when your local kineticist comes into the picture. You ask: how is the concentration of the reactant and product changing in time? The kineticist will go in the lab, make measurements, and provide you with curves that look like those shown in Fig. 1.1. These track the evolution of the concentrations of A and B, at a given temperature T and pressure p. If T and p are changed, the curves will be different.
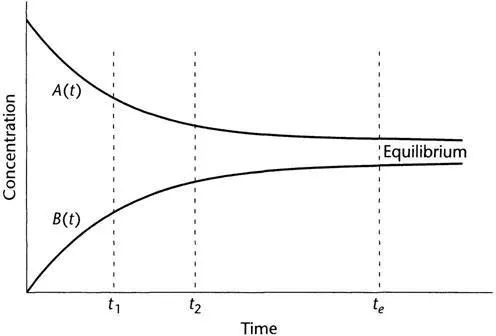
These curves can be used to calculate how much B is formed if you allowed the reaction to go on for a time t1 < te. The reaction has not reached equilibrium at t1 (Fig. 1.1), but it produced a considerable amount of B. You can calculate how much B is made per clay, if the reactor is purged at time t1 or t2 or t3 … after the reaction started, and then you load and repeat the operation all day. In this way it is possible to find the most profitable time for stopping the reaction.
At a different temperature or pressure the most profitable time will be different. I have to call my kineticist again and ask for new curves, for all pressures and temperatures worth considering. Now I can pick the most profitable temperature, pressure, and stopping time.
This is not exactly how plant design proceeds, but it gives the general idea: no matter how your process is designed, the kineticist is a central character in this drama.
Perhaps you are not interested in the chemical industry: your passion is biochemistry. As I type these lines, thousands of chemical reactions are going on in my body. Some are there just to keep me alive. Others ensure that I will be alive tomorrow. Some allow me to formu...
Table of contents
- Cover
- Half Title
- Title Page
- Copyright Page
- Table of Contents
- Preface
- How to use the workbooks, exercises, and problems
- Chapter 1 Generalities about the rates of chemical reactions
- Chapter 2 Irreversible first-order reactions
- Chapter 3 The temperature dependence of the rate constant: the Arrhenius formula
- Chapter 4 Irreversible second-order reactions
- Chapter 5 Reversible first-order reactions
- Chapter 6 Reversible second-order reactions
- Chapter 7 Coupled reactions
- Chapter 8 An example of a complex reaction: chain reactions
- Chapter 9 Enzyme kinetics
- Further reading
- Index