Exposure to chemicals is unavoidable–we live in a chemical world. We are composed of, and, in the course of our daily lives, exposed to, a wide range of chemicals, the vast majority of which are naturally occurring. However, an increasing number of those found in our bodies are persistent man-made chemicals such as siloxanes, polychlorinated biphenyls, or bisphenol A. The substances of concern themselves vary over time and are dependent on popular anxiety; in previous times, organochlorine pesticides such as DDT and its metabolite DDE were the substances of concern, but as their use as fallen, so has the general fear of exposure. It is a comforting and often-held belief that all man-made chemicals are poisonous and all natural chemicals are safe. Sadly, this is not the case; for example, botulin toxin, the active principle in Botox injections, is one of the most poisonous chemicals known but is found naturally. The ancient Greeks and Romans killed each other with natural poisons such as hemlock. Lead and cadmium are natural elements but with no levels that can be described as safe.
It is not simply these obvious toxicants, however, that should be considered to be poisonous. Caffeine is widely available and naturally occurring in coffee and tea, but is also increasingly an ingredient in energy drinks. Consumers of these drinks may not be aware of this and may overdose on caffeine without intending to. While caffeine may be tolerable at relatively high doses in humans, pets such as dogs may not tolerate such doses. In a similar vein, some dogs are more sensitive than others to the effects of chocolate and grapes, both of which can be very toxic to them. While it is easy to understand that something like coffee can have adverse effects if consumed to excess, extrapolating the same concept to water may appear more difficult. However, it has been realized that drinking water to avoid dehydration during marathons can result in overcompensation by the runners, which is then followed by toxicity due to overconsumption of water.
The idea that an exposure to a chemical or substance may lead to toxicity is not a new concept. The furore over the presence of lead in the paint for children’s toys was presciently foreseen in the Treatise on Adulterations by Frederick Accum, published in 1820. In a concluding paragraph, he observed that children are apt to mouth any toys or objects and that the practice of painting toys with poisonous coloring substances should be abolished. This is so obviously self-evident that it is a wonder that action has been taken only recently and, even then, is effective only by postmanufacture testing.
Toxicology is a very broad discipline, requiring broad expertise in a number of areas, including chemistry, pharmacology, physiology, biochemistry, anatomy, and numerous others. Definitions of toxicology tend to emphasize the role of exogenous substances or xenobiotics (literally foreign chemicals), while implicitly ignoring the contribution to toxic effect that can be seen with endogenous substances. The overproduction of endorphins in athletes and resulting runners’ high is an example of this; the storage of various proteins in Alzheimer’s disease is another. While many drug development candidates are clearly nonendogenous (xenobiotic), it may be prudent to consider unnaturally high concentrations of an endogenous substance in an unusual location to be xenobiotic too. It should also be considered that toxicity of a substance varies from subject to subject and a toxic reaction can occur from exposure to a normally nonnoxious substance, such as peanuts.
Absence of a chemical (see Focus Box 1.1 for terminology) can also have an effect; vitamin deficiency or decreased sensitivity to insulin (or its reduced production) may also be seen as an effect associated with chemicals. Liebler has written a broad-based review that considers the place of toxicology in the wider context of health and medicine, and also considers the role of endogenous chemicals (Liebler 2006). He points out that the implicit link between toxicology and exposure to xenobiotics ignores the role of endogenous chemicals and produces an unwarranted separation between toxicology, and health and medical practice (although the role of the occupational toxicologist comes closer to this than other branches of the science). Endogenous substances are important in disease but can also be generated in response to xenobiotic exposure. While we can control exogenous exposures to a certain extent, some more than others, there is no easy escape from disease such as cancer, diabetes, and others that can be related to diet, lifestyle, and so forth.
Toxicology is a science that has a direct impact on, and responsibility to, the public in a way that other subjects, for instance, astronomy or particle physics, do not. This responsibility arises from the role that toxicology has in assessing the safety of chemicals that have been or will be in daily use or to which the public are exposed. If the assessment is wrong, there is a distinct probability that adverse effects will be seen in exposed populations or that the benefits of a new chemical will be denied to people who would be advantaged by its use.
The public perception of toxicity is very important to people who conduct or interpret toxicological investigations. A change may be incorrectly perceived as adverse through incomplete access to all information, and this will provoke questions: When is a cluster of disease patients significant? How do you investigate? Whom should we believe? Why? What is the true, unprejudiced significance of this finding for the exposed population? Major concerns of the public are cancer, loss of special senses (especially sight), general debilitation, reproductive effects, disease, and shortened life span. Frame of reference is everything; much emphasis is placed on exposure to pesticides in food, without concern about the natural chemicals that occur in the same plants (e.g., green potatoes or broccoli), or on exposure to low-level radiation but not on sunbathing and consequent increased risk of skin cancer, including melanoma.
How a problem is expressed is also very important. Often, communication of toxicological information is one sided, emphasizing the apparently beneficial elements, while ignoring others. For instance, if the incidence of a particular fatal disease is 20 people in 1000, and this could be reduced by a novel (possibly hazardous) treatment by 20%, the new incidence would be 16 in 1000. However, the initial situation is that 98% of people are free of the disease; the new treatment would increase that to 98.4%. An increase of 0.4% is much less attractive than a decrease of 20%.
Accidents and emergencies, whether involving human, animal, or plant life, often provide salutary lessons. After the discharge of dioxins at Seveso, Italy, in 1976, prolonged investigations told us that dioxin is very toxic to animals in various ways; it is clear that humans suffer chloracne but other effects in humans are unproven or unknown. In another example, the discharge of inorganic mercury waste at Minimata Bay in Japan shows that nature does not always make things safer–in fact, quite the opposite: it can increase hazard, in this case, by methylation and increasing lipophilicity of the mercury, such that the human food chain was affected. It is automatically assumed by many that synthetic chemicals are harmful, but this assumption may ignore significant benefits.
More recently, pet food and infant formula were deliberately adulterated with melamine, which resulted in kidney toxicity and a number of deaths in both pets and human consumers, including children.
In the developed world, pharmaceutical standards and purity are assumed and are regulated; however, these same standards are not applied to the quality of designer drugs or the diluent of street cocaine. The expanding market for herbal extracts and remedies provides real cause for concern; for example, are the sources correctly identified, processed, stored, and labeled?
Treatment of some foods, such as peanuts, with mold-preventing chemicals carries some small risk from the chemical exposure, but importantly, the absence of mold markedly reduces the risk of liver cancer due to aflatoxin, which is produced by Aspergillus flavus growing on damp-stored peanuts. Aflatoxin is a particularly potent hepatotoxin and carcinogen, which may induce cancer at levels as low as 1 ppb; it has been found in trace amounts in peanut butter prepared from untreated peanuts. This could be sold as “organic” peanut butter; does this support the campaign for organic production?
THE BEGINNINGS OF TOXICOLOGICAL MEASUREMENT
Although not always known as toxicology, this fascinating amalgam of different disciplines has had a long history, stemming from the Ebers Papyrus of the ancient Egyptians and progressing steadily through ancient Greece and Rome. In Greece and Rome, the knowledge of poisons was crucial in eliminating unwanted politicians, rivals, or relatives. This use was particularly noted in some Roman wives who used contract poisoners to do away with rich husbands so that they could inherit the wealth and move on to the next hapless, but temptingly rich, victim. This cheerful habit was revived in Renaissance Italy, where dwarves were created by feeding known growth inhibitors to children, a practice noted by Shakespeare in A Midsummer Night’s Dream, where he writes, “Get you gone, you dwarf … of hindering knotgrass made.”
It was clear to the practitioners of the day that the dose, that is, the level of exposure, was the critical factor determining success or failure. However, it was Paracelsus, born in 1493, who linked dose with effect by stating that everything is a poison; only the dose differentiates between a poison and a remedy. Although this has been quoted extensively, the full context of the quotation is instructive (Ball 2006). The religious context is clear, as Paracelsus asks what God has created that was not blessed with some gift beneficial to man. Possibly without appreciating its latter-day significance, he says that to despise a poison is to be ignorant of what it contains, which might be interpreted as indicating ignorance of potential toxicity or therapeutic benefit. Having said that, it has to be admitted that, while his treatments did not have the precision that he may have wanted, he was at the forefront of the movement to formulate new medicines.
To put dose relationship in a modern context, a daily glass of red wine may be considered to be therapeutically beneficial (depending on which epidemiological study you wish to believe); increase that to a bottle or more a day, and cirrhosis of the liver beckons.
It is usually fairly easy to say what dose of a chemical is toxic or harmful but much more difficult to predict safety. In fact, as it is not possible to prove a negative, the question “Is it safe?” is effectively impossible to answer affirmatively as biological responses differ between individuals and some may respond to low levels of a chemical when the majority are unaffected. While the concept of poisonous was understood, for instance, in the seventeenth century, as the effect of poisons or of an excess of something, the concept of safety was of little concern. The work of people like Percival Potts, who linked scrotal cancer in former chimney sweeps with prior exposure to soot, led to gradual recognition of safety as a concept. However, it took many years to do anything about it, in line with the speed of change in regulation in the twenty-first century.
With the enormous increase in the use of chemicals that has taken place during the late nineteenth century and in the twentieth century, it became apparent that there should be an increasing emphasis on demonstration of safety. This concern for safety is applied in many areas, ranging from novel or genetically modified (GM) foods to industrial chemicals and from pharmaceuticals to leachables from medicinal packaging. In some cases, where a traditional or long-used chemical is known to be unsafe, efforts are made to find a substitute. When the search is successful, it is sometimes the case that the substitute removes the old problems while introducing new ones. However, it is generally accepted that to predict safety, given that there is no such thing as a safe chemical or a risk-free existence, it is necessary first to demonstrate what dose of the study chemical is toxic and how that toxicity develops as dose increases.
In the modern context, there is public recognition that there are chemicals to which people are exposed voluntarily (for example, cigarette smoke, medicines, and alcohol) and those to which exposure is involuntary (pesticides in vegetables, other people’s cigarette smoke, pollution, food preservatives, antibiotics in food animals, and so on). There is a lively public debate on many of these substances, which often takes extreme views due to lack of knowledge or willful misinformation or misinterpretation by interested parties. Such fine lines are drawn by politicians, but it is the responsibility of the toxicological community to define safe doses or inclusion limits for these various chemicals. Above all, this must be done in a credible manner, within the existing framework of regulation and ethical behavior.
In addition, there is a growing body of scientific work investigating the effects of chemicals that occur naturally in our food. For instance, it has been shown in several papers that some constituents of mushrooms can cause cancer in mice when given at high dosages. It should be borne in mind that, in the correct circumstances, administration of water might be capable of inducing cancer in mice (although it is more often a cause of drowning). If a study was conducted that demonstrated that water was a carcinogen, would this mean that we should give up drinking water or, maybe, convert it to beer? The relationship between dosage and harmful effects is crucial in the assessment of chemicals, including those that occur in a natural diet. Given that much of the exposure of people to individual chemicals is at low levels, the fact that many may cause cancer at high levels is probably not significant for everyday life. Furthermore, it is important to remember that the majority of testing is performed on single substances, whereas the majority of exposure is to many substances simultaneously, for example, in a normal diet. Life is about mixtures.
STRONG TOXICANTS AND WEAK TOXICANTS
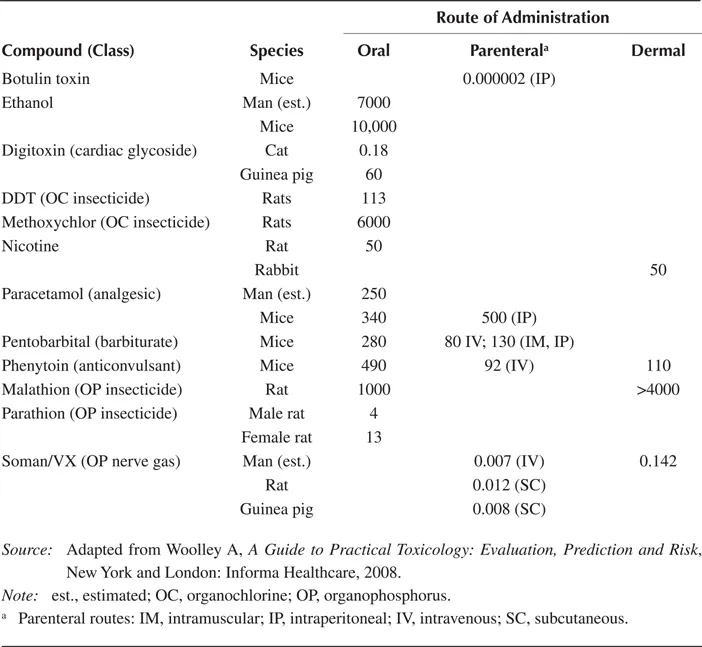
A reasonably clear ranking of potency among chemicals can be established when appropriate compounds are selected for comparison (Table 1.1). Thus, tetrachlorodibenzo-p-dioxin (TCDD) is one of the most potent chemicals known, and can be lethal to guinea pigs at 1 μg/kg of body weight, while the lethal dose of an everyday substance such as paracetamol (acetaminophen) is very much higher. However, this type of ranking is, in some ways, distorting, as the potency of any chemical can change markedly depending on the species under consideration, TCDD being 20 to 50 times less toxic in rats (Table 1.2) (Russell and Burch 1959). Organophosphate insecticides are much more toxic, by design, in insects than in mammals. Table 1.1 also shows the differing toxicities according to species and route of administration.