
- 426 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Radiation Safety in Radiation Oncology
About this book
The proposed book aims to explain the basic principles, concepts and regulations behind radiation protection and their application in the field of radiation oncology practice. This book will be useful to all those students, teachers and practicing professionals involved in the field of radiation oncology.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Radiation Safety in Radiation Oncology by K. N. Govinda Rajan in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Oncology. We have over one million books available in our catalogue for you to explore.
Information
1
Basic Atomic and Nuclear Physics
1.1 Introduction
We all have a basic idea about the atom as resembling the planetary model. There is a small nucleus with dimensions of the order of 10−13 cm, with electrons swirling around the nucleus like the planets around the sun. The size of the atom is larger by about 105 times compared to nuclear dimensions. Though the quantum mechanical idea of an atom is not that simplistic, this classic picture explains many of the characteristics of the atom, and we will make use of this to understand the observed properties of the atom.
An atom is electrically neutral and hence the nuclear charge must be equal to the total electronic charge. The nucleus contains neutrons and protons carrying a charge of 0 and +1, respectively, expressing the charge in terms of e = 1.6 × 10−19 C. The charge of the electron is −1. The neutrality of the atom suggests that the number of electrons must be equal to the number of protons in the nucleus.
The mass of atoms is minute and so the usual units like grams or kilograms are not suitable units to express them. The unit of mass must be the mass of the atom itself, so that the atomic masses can make more sense.
1.2 A Short Introduction to Atomic Structure
Niels Bohr, in his atom model proposed in 1913, introduced the concept of quantization (or discreteness of matter at submicroscopic level), to derive the discrete energy levels of the hydrogen atom. This could successfully explain the experimentally observed spectral lines of hydrogen. The energy levels corresponded to the atomic shells named K, L, M, etc., K being the first shell of lowest energy, L the second shell of higher energy, and so on. The shells were characterized by a principal quantum number n which assumed integer values (n = 1 for K shell). The transitions between the atomic levels gave rise to the emission of discrete spectral lines in the visible or ultraviolet regions, or in the X-ray region, depending on the strength of binding and the energy level differences. An atom, in its ground state, is in its lowest energy state (i.e., occupies the orbitals closest to the nucleus) according to the rules governing their occupancy. The unoccupied energy levels are vacant in the ground state. When the electrons of an atom absorb energy, they go to the higher energy states. The electrons quickly (in about 10−8 seconds) return to the lower vacant states emitting the excess energy in the form of photons. The transitions occur because the electrons would like to be closest to the nucleus for maximum binding.
When the energy supplied is greater than the binding energy (BE) of the electron, the electron can be knocked out of the atom—this is called ionization—and the atom becomes an ion. The electron will exist in its lowest energy state if all the electrons occupy the first shell. However, Pauli’s exclusion principle forbids all electrons to occupy the same state. According to this principle, no two electrons (or nucleons) in an atom can have identical quantum numbers.
NOTE: Since the electrons can exist in two spin states (spin up or spin down) each orbital can be occupied by not more than a pair of electrons in opposite spin states. The atomic particles have half integral spin = ½ (expressed in units of ℏ). These particles follow the Pauli exclusion principle. This principle is the most fundamental principle followed by atomic particles.
We will briefly see how the electrons are organized in an atom, as per the above principle.
In an atom, the atomic shells are divided into subshells and the subshells are further divided into orbitals. Quantum mechanics determines the configuration of an atom and we will not get into its details. We will only look at it in a qualitative way. The easiest way to visualize all the orbitals is as follows:
- Shell quantum number = n (takes values 1, 2, 3, …).
- Subshell quantum number = l = 0, 1, 2, … (n−1). The electron energy depends on n and l values.
For each l, orbital quantum number ml = +l to –l = (2l + 1) orbitals; Δml = ±1.
This gives the number of orbitals in the subshell and can be thought of as different orientations of the orbitals. These orbitals are degenerate (meaning having the same energy) but in a magnetic field, the degeneracy will be removed and the energy levels split up to show a fine structure.
Number of electrons in each orbital = 2 (spin up and spin down).
In spectroscopy, the subshells are referred to as s, p, d, f, (referring to sharp, principal, diffuse, fundamental series, respectively). Beyond f, the letters g and h are used for the subshells.
Example 1.1
How many electrons are in the shell n = 2?
Subshells: l = 0 and 1 (s and p)
No. of electrons in s state = 2 (ml = 0)
For the p subshell, there are three orbitals (ml = 1, 0, −1).
The number of electrons in the p subshell = 3 × 2 = 6.
Therefore, shell two can contain eight electrons.
The electron configuration is written as follows: 1s2 refers to s state containing two electrons. If the first and second shells are full, the configuration will be 1s22s22p6. So, it is easy to write the electron configuration of atoms. The simple formula 2n2 gives the maximum number of electrons a shell of principal quantum number n can take.
1.3 The Filling of Sublevels
In the hydrogen atom model, the electron energy is governed only by n. In the case of multiple electron atoms, the subshells do not have the same energy, as can be seen from the energy level diagram. This is because of the mutual repulsive interaction between the electrons. The energy of the subshell gradually increases with increasing l value. However, the orbitals in a subshell have the same energy, as stated earlier (see Figure 1.1).
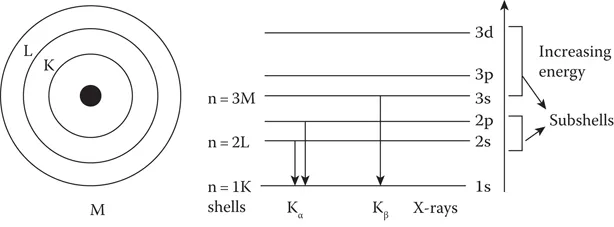
FIGURE 1.1
Atomic shells, energy levels, and transitions.
The unpaired electrons have less energy compared to paired electrons, so they tend to occupy unoccupied orbitals first before they start pairing. There is an overlapping of the orbital energy levels of shells for higher Z elements, since the atomic energy levels are not decided by just the n value, but by n + l values. Figure 1.2 shows the paired electrons in the orbitals and the overlapping of higher energy states.
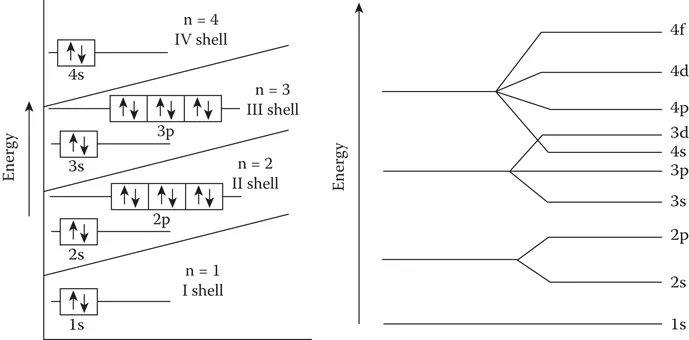
FIGURE 1.2
Energy levels in low Z and high Z atoms.
The orderly filling of atomic levels takes place up to the first 18 elements, beyond which the overlapping occu...
Table of contents
- Title Page
- Copyright Page
- Dedication
- Table of Contents
- Foreword
- Preface
- Acknowledgments
- Author
- 1. Basic Atomic and Nuclear Physics
- 2. Basic Medical Radiation Physics
- 3. Evolution of Radiation Protection and Radiation Risk Concepts
- 4. Radiation Protection Quantities, Units, and Standards
- 5. System of Radiation Protection and Regulations in Radiation Oncology
- 6. Calibration of Radiation Monitoring Instruments
- 7. Radiation Detectors for Area (Ambient) Monitoring
- 8. Radiation Detectors for Individual Monitoring
- 9. Transport of Radioactive Materials in Radiation Oncology
- 10. Radiation Protection in External Beam Therapy
- 11. Radiation Protection in Brachytherapy
- 12. Prevention of Accidents in Radiation Oncology
- Index