
- 270 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
About this book
The first edition of this book covered the basic treatment of the enzyme reaction using the overall reaction kinetics and stopped-flow method, the general properties of protein and cofactors, the control of enzyme reaction, and the preparation of enzyme protein. These topics are the basis of enzyme research and thus suitable for the beginner in the field. The second edition presents the cofactors produced via the post-translational modification of the enzyme's active site. These cofactors expand the function of enzymes and open a new research field. The carbonyl reagent phenylhydrazine and related compounds have been useful in finding some of the newly discovered cofactors and thus have been discussed in this edition. The topic of the control of enzyme activity through the channel of substrates and products in polyfunctional enzymes has also been expanded in this book.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
Chapter 1
Introduction
1.1 General Properties of Enzyme
1.1.1 Enzyme Specificity
1.1.2 Rate Enhancement
(1.1) |
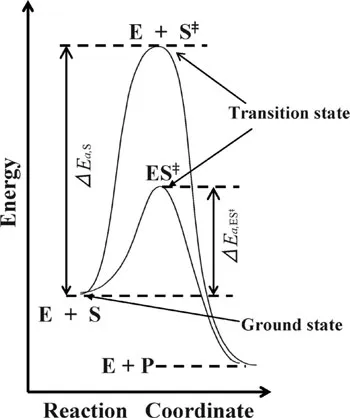
(1.2) |
(1.3) |
Enzymes | Rate enhancement |
|---|---|
Adenosine deaminase | 2.1 × 1012 |
Alkaline phosphatase | 1.6 × 1016 |
Alkylsulfatase | 2 × 1026a |
Chorismate mutase | 1.9 × 106 |
Peptidase | 1.5 × 1010 |
Triose phosphate isomerase | 6.2 × 109 |
Urease | 2.3 × 1013 |
Abzyme (amide hydrolysis) | 2.5 × 105 |
1.2 Examples of Enzyme
1.2.1 N...
Table of contents
- Cover
- Half Title
- Title Page
- Copyright Page
- Table of Contents
- Preface
- 1. Introduction
- 2. Overall Reaction Kinetics
- 3. Factors That Affect Enzyme Activity
- 4. Effect of pH, Temperature, and High Pressure on Enzymatic Activity
- 5. Measurement of Individual Rate Constants
- 6. Structure of Protein
- 7. Cofactors
- 8. Search of Active Site
- 9. Control of Enzyme Activity
- 10. Channeling of Substrates and Products
- 11. Preparation of Enzyme
- 12. A Case Study: L-Phenylalanine Oxidase (Deaminating and Decarboxylating)
- Appendix
- Solutions
- Index
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app