
- 384 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
Using Geochemical Data brings together in one volume a wide range of ideas and methods currently used in geochemistry, providing a foundation of knowledge from which the reader can interpret, evaluate and present geochemical data.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
Chapter 1

Geochemical data
1.1 Introduction
This book is about geochemical data and how they can be used to obtain information about geological processes. Conventionally geochemical data are subdivided into four main categories: the major elements, trace elements, radiogenic isotopes and stable isotopes (see Table 1.1). These four types of geochemical data each form the subject of a chapter of this book. Each chapter shows how the particular form of geochemical data can be used and how it provides clues to the origin of the suite of rocks in question. Different methods of data presentation are discussed and their relative merits evaluated.
Table 1.1 Whole-rock geochemistry of komatiite flows from the Belingwe greenstone belt, Zimbabwe (Data from Nisbet et al., 1987)
ZV14 | ZV85 | ZV10 | |
Major element oxides (wt%) | |||
SiO2 | 48.91 | 45.26 | 45.26 |
TiO2 | 0.45 | 0.33 | 0.29 |
Al2O3 | 9.24 | 6.74 | 6.07 |
Fe2O3 | 2.62 | 2.13 | 1.68 |
FeO | 8.90 | 8.66 | 8.70 |
MnO | 0.18 | 0.17 | 0.17 |
MgO | 15.32 | 22.98 | 26.21 |
CaO | 9.01 | 6.94 | 6.41 |
Na2O | 1.15 | 0.88 | 0.78 |
K2O | 0.08 | 0.05 | 0.04 |
P2O5 | 0.03 | 0.02 | 0.02 |
S | 0.04 | 0.05 | 0.05 |
H2O+ | 3.27 | 3.41 | 2.20 |
H2O− | 0.72 | 0.57 | 0.28 |
CO2 | 0.46 | 0.84 | 1.04 |
Total | 100.38 | 99.03 | 99.20 |
Selected trace elements (ppm) | |||
Ni | 470 | 1110 | 1460 |
Cr | 2080 | 2770 | 2330 |
V | 187 | 140 | 118 |
Y | 10 | 6 | 6 |
Zr | 21 | 16 | 14 |
Rb | 3.38 | 1.24 | 1.38 |
Sr | 53.3 | 32.6 | 31.2 |
Ba | 32 | 12 | 10 |
Nd | 2.62 | 1.84 | 2.31 |
Sm | 0.96 | 0.68 | 0.85 |
Radiogenic isotope ratios | |||
εNd | +2.4 | +2.4 | +2.5 |
87Sr/86Sr | 0.7056 | 0.70511 | 0.70501 |
Stable isotope ratios (‰) | |||
δ18O | +7.3 | +7.0 | +6.8 |
Note: Major elements and Ni, Cr, V, Y, Ba determined by XRF; FeO determined by wet chemistry; H2O and CO2 determined by gravimetry; Rb, Sr, Sm, Nd determined by IDMS.
The major elements (Chapter 3) are the elements which predominate in any rock analysis. They are Si, Ti, Al, Fe, Mn, Mg, Ca, Na, K and P, and their concentrations are expressed as a weight per cent (wt %) of the oxide (Table 1.1). Major element determinations are usually made only for cations and it is assumed that they are accompanied by an appropriate amount of oxygen. Thus the sum of the major element oxides will total to about 100 % and the analysis total may be used as a rough guide to its reliability. Iron may be determined as FeO and Fe2O3, but is sometimes expressed as ‘total Fe’ and given as either FeO(tot) or Fe2O3(tot).
Trace elements (Chapter 4) are defined as those elements which are present at less than the 0.1 % level and their concentrations are expressed in parts per million (ppm) or more rarely in parts per billion (ppb; 1 billion = 109) of the element (Table 1.1). Convention is not always followed however, and trace element concentrations exceeding the 0.1 % (1000 ppm) level are sometimes cited. The trace elements of importance in geochemistry are identified in Table 1.5 and shown in Figure 4.1.
Table 1.5 Elements readily analysed by XRF, INAA, IDMS, AAS, ICP and ICP-MS
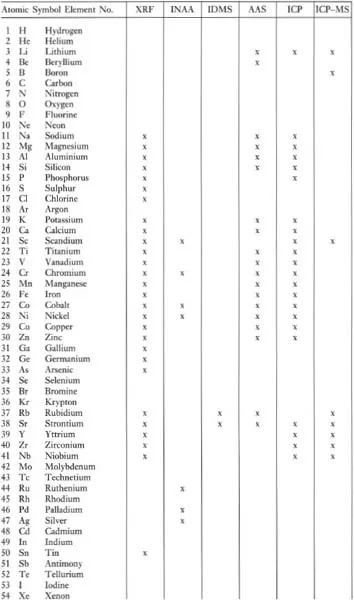
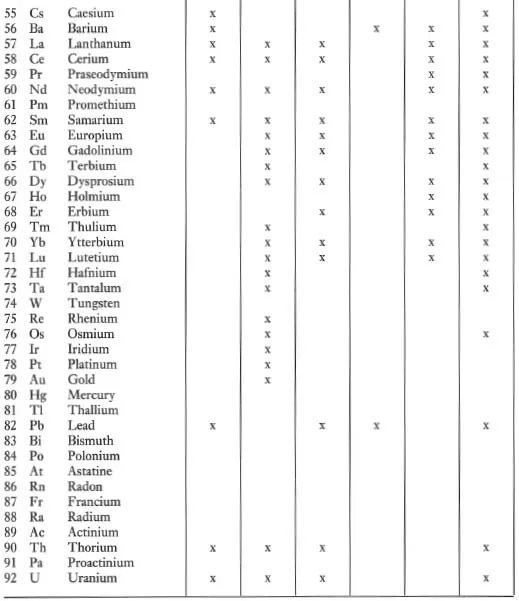
Some elements behave as a major element in one group of rocks and as a trace element in another group of rocks. An example is the element K, which is a major constituent of rhyolites, making up more than 4 wt % of the rock and forming an essential structural part of minerals such as orthoclase and biotite. In some basalts, however, K concentrations are very low and there are no K-bearing phases. In this case K behaves as a trace element.
Volatiles such as H2O, CO2 and S are normally included in the major element analysis (Table 1.1). Water combined within the lattice of silicate minerals and released above 110 °C is described as H2O+. Water present simply as dampness in the rock powder and driven off by heating below 110 °C is quoted as H2O− and is not an important constituent of the rock. Sometimes the total volatile content of the rock is determined by ignition at 1000 °C and is expressed as ‘loss on ignition’ (Lechler and Desilets, 1987).
Isotopes are subdivided into radiogenic and stable isotopes. Radiogenic isotopes (Chapter 6) include those isotopes which decay spontaneously due to their natural radioactivity and those which are the final daughter products of such a decay scheme. They include the parent-daughter element pairs Rb–Sr, Sm–Nd, U–Pb, Th–Pb and K–Ar. They are expressed as ratios either in absolute terms 87Sr/86Sr (e.g.) or relative to a standard (the ε-notation) (Table 1.1).
Stable isotope studies in geology (Chapter 7) concentrate on the naturally occurring isotopes of light elements such as H, O, C and S which may be fractionated on the basis of mass differences between the isotopes of the element. For example, the isotope 18O is 12.5 % heavier than the isotope 16O and the two are fractionated during the evaporation of water. Stable isotopes contribute significantly to an understanding of fluid and volatile species in geology. They are expressed as ratios relative to a standard using the δ-notation (Table 1.1)
The major part of this book discusses the four main types of geochemical data outlined above and shows how they can be used to identify geochemical processes. In addition, Chapter 5 has been included to show the way in which trace and major element chemistry is used to determine the tectonic setting of some igneous and sedimentary rocks. Chapter 2 discusses some of the particular statistical problems which arise when analysing geochemical data-sets, and some recommendations are made about permissible and impermissible methods of data presentation.
In this introductory chapter we consider three topics: (1) the geochemical processes which are likely to be encountered in nature and their geochemical signatures; (2) the interaction between geological fieldwork and the interpretation of geochemical data; and (3) the different analytical methods currently in use in modern geochemistry.
1.2 Geological processes and their geochemical signatures
A major purpose of this text is to show how geochemical data can be used to identify geological processes. In this section the main geochemical signatures of igneous, sedimentary and metamorphic processes are briefly summarized and presented in graphical (Figures 1.1 to 1.3) and in tabular form (Tables 1.2 to 1.4). This brief survey is augmented by fuller discussions elsewhere in the text. Each of the Tables 1.2 to 1.4 lists the geological processes which may have a geochemical signature and identifies the sections in the book where the particular process is described and characterized using major or trace elements, and radiogenic or stable isotopes.
Table 1.2 The geochemical signatures of igneous processes identified by section in subsequent chapters
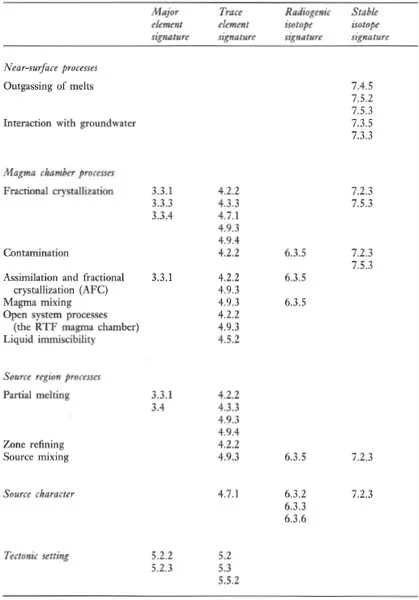
Table 1.3 The geochemical signatures of sedimentary processes, identified by section in subsequent chapters
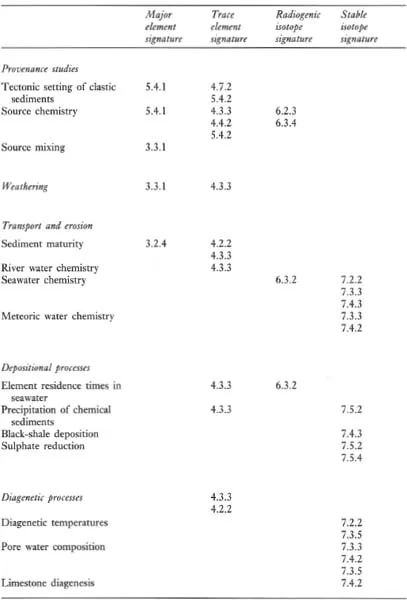
Table 1.4 The geochemical signatures of metamorphic processes, identified by section in subsequent chapters
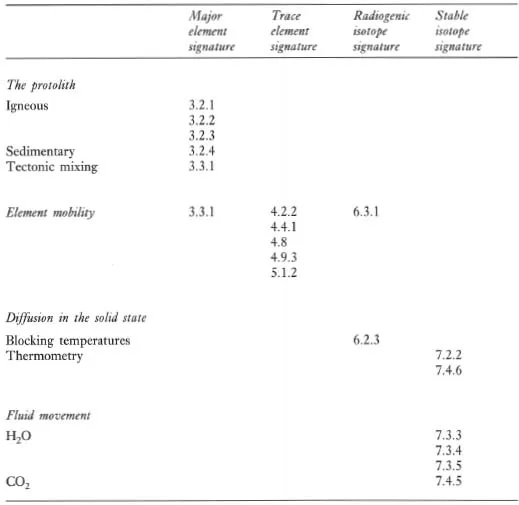
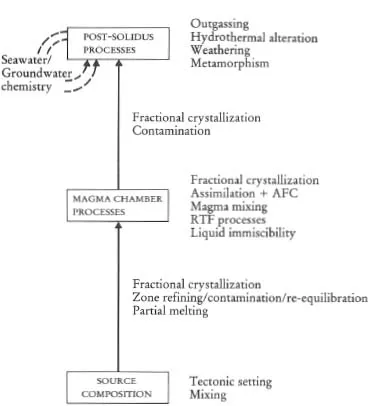
Figure 1.1 Flow diagram showing the principal processes which control the chemical composition of igneous rocks.
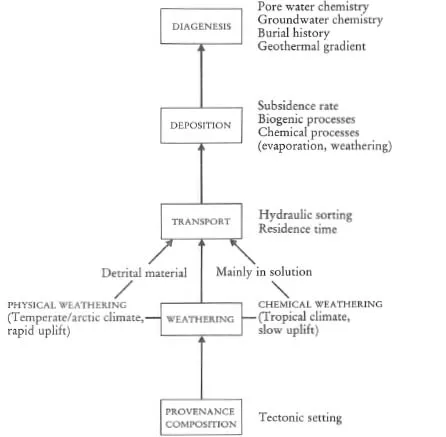
Figure 1.2 Flow diagram showing the principal processes which control the chemical composition of sedimentary rocks.
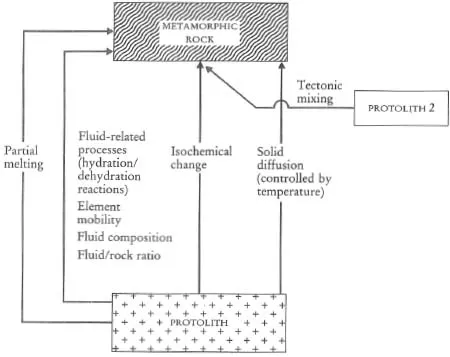
Figure 1.3 Flow diagram showing the principal processes which control the chemical composition of metamorphic rocks.
1.2.1 Processes which control the chemical composition of igneous rocks
The chemical composition and mineralogy of the source region exerts a fundamental control over the chemistry of magmatic rocks. The major and trace element composition of a melt is determined by the type of melting process and the degree of partial melting, although the composition of the melt can be substantially modified en route to the surface (Figure 1.1). The source region is best characterized by its radiogenic isotope composition because isotope ratios are not modified during partial melting and magma chamber processes. The composition of the source itself is a function of mixing processes in the source region. This is particularly pertinent to studies of the mantle, and in the last decade important advances have been made in understanding mantle dynamics through the isotopic study of mantle-derived oceanic basalts (see Section 6.3.6).
Most magmatic rocks are filtered through a magma chamber prior to their emplacement at or near the surface. Magma chamber processes frequently modify the chemical composition of the primary magma, produced by partial melting of the source, through fractional crystallization, magma mixing, contamination or a dynamic mixture of several of these processes. Resolving the chemical effects of these different processes requires the full range of geochemical tools — major and trace element studies coupled with the measurement of both radiogenic and stable isotope compositions. Excellent and detailed discussions of magma chamber processes are given by Hall (1987 — Chapter 7) and Wilson (1989 — Chapter 4).
Following emplacement or eruption, igneous rocks may be chemically modified, either by ...
Table of contents
- Cover
- Half Title
- Title Page
- Copyright Page
- Dedication
- Table of Contents
- Preface
- Acknowledgements
- Glossary
- 1. Geochemical data
- 2. Analysing geochemical data
- 3. Using major element data
- 4. Using trace element data
- 5. Discriminating between tectonic environments using geochemical data
- 6. Using radiogenic isotope data
- 7. Using stable isotope data
- References
- Index
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Using Geochemical Data by Hugh R. Rollinson in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Geography. We have over one million books available in our catalogue for you to explore.