
- 488 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Steel Carriage by Sea
About this book
Steel: Carriage by Sea provides invaluable information on how to prevent claims arising when transporting steel, including careful handling, good stowage and care of cargo throughout its entire journey. This book covers every aspect of the transportation and surveying of steel products carried on ships. The fifth edition provides practical advice on: • How to prevent damage to steel cargoes • How to deal with subsequent claims • The different types of steel products manufactured and their particular packing requirements • How the various types of steel products should be loaded, stowed, lashed, secured and ventilated aboard a ship • Maintenance of the ships' hatchover, tanktop strength and cargo documentation • The surveying and claims handling of the various typesof steel products • The corrosion process of steel
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Steel Carriage by Sea by Arthur Sparks in PDF and/or ePUB format, as well as other popular books in Law & Law Theory & Practice. We have over one million books available in our catalogue for you to explore.
Information
Chapter 1
Manufacturing of Iron and Steel
The Manufacturing of Iron
Iron is obtained from iron ore, which is principally a chemical compound of iron and oxygen; it contains minor amounts of other elements and also unwanted matters such as rock, clay and sand. It has been estimated that 5% of the earth’s crust is composed of iron. Iron ores from Europe are, in most instances, weak ores containing about 25% pure iron, and the mining and extraction of the iron from such ores is very costly. High-grade ores in abundant quantities are found in Canada, South America, Africa, India, Australia and Sweden. These high-grade ores are shipped around the world using large bulk carrier ships carrying up to 300,000 metric tons of the ore.
The following, owing to their high yield of iron, are the important iron ores:
| Magnetite | (Fe3O4) | 72% iron (black ore) |
| Haematite | (Fe2O3) | 70% iron (red ore) |
| Limonite | (Fe2O3H2O) | 60% iron (yellow brown ore) |
| Siderite | (FeCO3) | 48% iron (light grey/brown) |
Note: Percentages of iron are approximate.
The uses for pure iron are few as its mechanical properties are unsuitable for general applications. Small amounts of other elements greatly modify its properties, the most important of which is carbon. The addition of a relatively small amount of carbon produces steel. By varying the carbon content, and other alloy additions, steels with a wide range of properties can be obtained.
The Blast Furnace
The blast furnace is used to extract iron from the iron ore and could be referred to as an indirect method of reduction to near pure iron. Sizes of the entire structure vary considerably. They are, however, of an immense size, reaching a height in many instances of more than 70 m with a diameter of 14 m. The furnace consists of a cylindrical shell constructed from steel plates, the inside walls of which are lined with heat-resistant firebricks (refractory bricks).
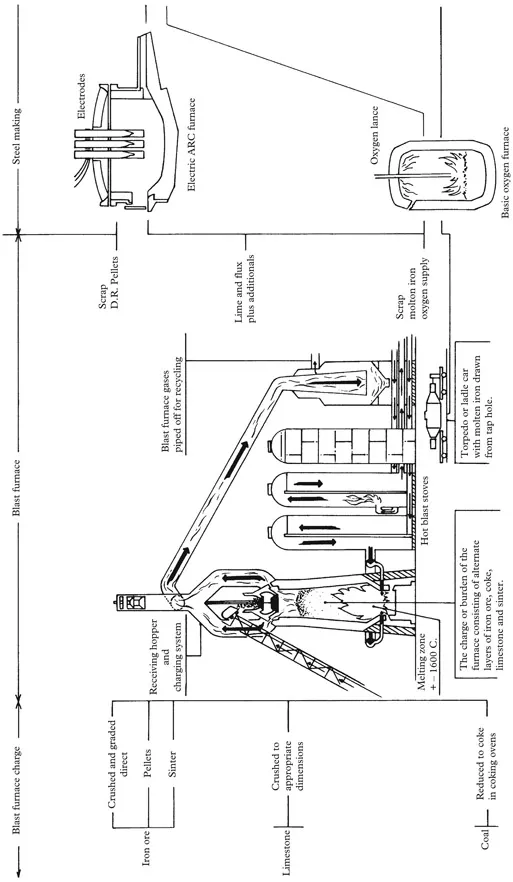
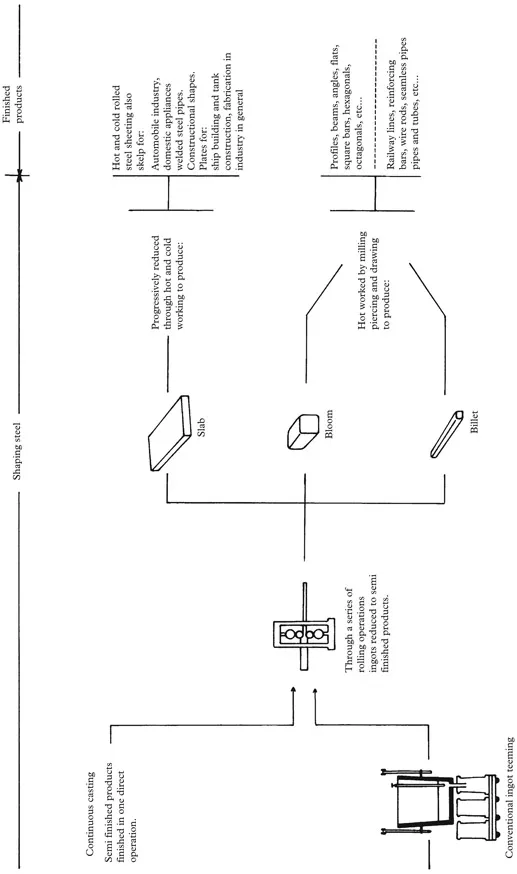
Fig. 1.1: Steel making—from iron ore to finished product
At the top of the furnace there is a double bell charging system through which the burden (or charge), consisting of iron ore, coke and limestone, is built up and consistently replenished.
The type of coke used in the production of iron is referred to as metallurgical coke. Such material is chosen owing to its low sulphur content, and coke may be looked upon as the skeleton of coal. Coke is produced from coal in special coking ovens normally installed at the steel works. In the blast furnace, coke has a triple role insofar as it is used as a fuel to raise the temperature within the furnace. It also physically supports the burden and its inherent porosity permits the gases to penetrate upwards to the top of the furnace. Its third function, which is of extreme importance, is that the carbon monoxide gases produced by burning off the coke combine with the oxygen in the iron ore, so reducing iron oxide to iron.
Adjacent to the aforementioned charging system are large exhaust pipes through which the hot gases rising to the top of the furnace are directed away into a dust cleaner, gas cleaning plant (spray chamber) and gas holder to be returned to heat the blast air (in the hot blast stoves) and eventually readmitted to the furnace, after which, these spent gases are exhausted to the atmosphere.
A blast furnace can operate continuously for up to five years and produce thousands of tons of iron per day. The uninterrupted iron producing period of the furnace is referred to as a campaign, which must, in due course, come to an end in order to renew the refractory linings which deteriorate as time goes by.
Simply explained, alternate layers of iron ore, coke and limestone are, as required, charged into the furnace through the double bell charging system. The hot blast of air introduced at the base of the furnace causes an extremely high temperature to be maintained within the furnace, as a result of which the coke burns and the carbon monoxide gases given off combine with the oxygen in the ore (iron oxide) to pass vertically upwards to the top of the furnace, and leave behind near molten iron. While this situation has been developing the limestone has scavenged the charge of extraneous materials, which accumulate in the form of slag.
Slag is composed of the impurities separated out from the ore to produce iron and this is achieved by the introduction of a flux to the furnace charge in the form of limestone. The slag is poured off through the slag hole or notch situated at the top level of the molten metal and retained for other uses. Although from the point of view of iron making, slag is a residual waste material, it is used in the building industry for the manufacture of insulation material and as a fertilizer, etc. Such goods are usually transported in jute bags and are odourless but very dusty.
The molten iron settles at the base of the furnace with the layer of molten slag on top. The molten iron, which is run off through a tap hole at the base of the furnace, plugged with fire clay and pierced at the appropriate time, is cast into pig iron or transported in its molten state direct to the converter for refining into steel. Many blast furnaces are located adjacent to a basic oxygen furnace and a rolling mill, thus becoming an integrated mill.
The furnace takes its name from the blast of hot air and gases forced up through the bottom of the furnace through the charge. Extremely high temperatures are involved and at the base of the furnace temperatures rise to more than 1,700°C. The temperature reduces gradually throughout the furnace to about 300°C at the top.
It is of interest to note that a medium-sized blast furnace can produce between 5,000 and 8,000 tons of iron in each 24-hour period, requiring about 7,500 tons of iron ore, 3,000 tons of coke, 750 tons of limestone and 2,000 tons of air.
The hot blast stoves are situated adjacent to, and are as high as, the blast furnace itself. Each stove is fitted with a brickwork system heated to a high temperature by circulating hot gases, piped into the stove from the exhausts at the top of the blast furnace. In such circumstances, the stove is said to be “on gas” until such time that the brickwork reaches a predetermined temperature. Thereafter, fresh air is piped into the stove, which is now “on air”, by a turbo blower heated to a high temperature through contact with the brickwork in the stove, later being blasted into the base of the furnace at temperatures of between 800 and 1,200°C. Three of these stoves are involved, each being alternately “on gas” and “on air”.
The molten iron, which melts at 1,540°C, is cast into either ingots or pig iron for the convenience of handling, storage and transportation. Ingots are large blocks normally trapezoidal in shape. They are intended for reheating and mechanical working to be reduced into semi-finished products such as slabs and blooms.
Pig iron or “pigs” is the basic raw material used to make steel and cast iron, and contains about 4% carbon, up to 3% silicon and also small quantities of sulphur, phosphorus and manganese. The name is derived from the fact that the molten iron is run off into channels which have branches, and in earlier days the outlay of this system, as viewed from above, could, by some stretch of the imagination, resemble newborn piglets suckling the sow, hence the name pig iron. Pig iron is in a suitable form for handling and storage purposes. The iron ingots or pigs are then further processed to produce either cast irons or steels. Common cast irons are principally iron, carbon and silicon alloys containing between approximately 2% and 4% carbon and 1% to 3% silicon. They can also contain small amounts of manganese, sulphur and phosphorus. The high carbon content makes the alloy brittle so that it cannot be rolled or forged and it is only suitable for casting. Some of the uses to which cast iron is suited are the manufacture of engine blocks, machine bases, gears, pipes, machine parts, etc.
The Manufacturing of Steel
There are essential two main methods of manufacturing the wide range of steels and steel alloys; they are the basic oxygen furnace (BOF) and the electric arc furnace (EAF). The EAF is normally associated with a “mini-mill”.
The feed for the BOF is normally pig iron and steel scrap and the feed for EAF is steel scrap and direct reduced iron (DRI). A brief description of these three products is provided below and the carriage of these products is described in Chapter 3.
Pig iron comes directly from the blast furnace and contains about 4% carbon, up to 3% silicon and also small quantities of sulphur, phosphorus and manganese. Pig iron is transported by sea, and in view of the fact that the goods have to be re-melted to the liquid state for the purpose of refining, no harm of a significant nature can be sustained by the material during normal transport conditions.
Steel scrap is carried by ships in huge amounts, often from Europe and North America to countries that cannot generate sufficient quantities of steel scrap to feed their local consumption/furnace capacity. Steel scrap comes in a number of forms and grades some of which require greater care during transportation than others.
DRI is almost pure iron (96% iron), which is produced in pellet, or briquette form by the direct reduction of iron pellets. It is free of tramp elements and is produced in such a physical form that it is energy efficient. There can be significant risks to vessels when shipping DRI in certain forms unless specific precautions are taken as detailed in the “Code of Safe Practice for solid bulk cargos” (BC code).
The Basic Oxygen Furnace
This is the main method of producing steel in bulk in tonnage terms, and the modern furnace can take about 40 minutes to convert iron and steel scrap into steel. The principal material used is hot metal, however: 70% molten iron and 30% scrap steel are often used together in this system.
The furnace is tilted and charged with steel scrap metal and then with molten iron, which is introduced directly from the blast furnace. The vessel is then returned to the upright position, after which a water-cooled oxygen lance is lowered into the furnace and oxygen is blown into the metal at great speed. The oxygen combines with carbon, sulphur, phosphorus and other elements, so reducing these unwanted impurities in the molten charge. During the oxygen blow period lime is added as a flux to help carry off oxidised impurities in the form of slag, which floats on the surface of the charge. The next step is to refine the metal by adding various elements in determined quantities in order that the desired composition of the steel is reached. Thereafter, the furnace is tipped into the horizontal position and the molten steel tapped off and run into a ladle. When all the steel has been removed, the furnace is tipped into an upside-down position and the slag is run off into a slag ladle.
The advantage of the basic oxygen furnace production of steel over previous methods, such as the open-hearth furnace, is that the pure oxygen used prevents nitrogen from remaining in the molten steel. Furthermore, the method affords high control over the quality of the steel produced.
The Electric Arc Furnace
The electric arc furnace is normally part of a mini-mill site. The “mini-mill” uses a method of producing steel that bypasses the necessity for using blast furnaces, iron ore, coking ovens, etc. For obvious reasons in recent years this method of steel production has become very popular. Naturally, as more and more of these mills are set up around the world, the demand for, and the price of, steel scrap will increase.
The electric arc process of making steel primarily uses steel scrap metal and direct reduced iron. One of the reasons for its popularity is the fact that it takes only approximately four hours to convert steel scrap/DRI into steel. The electric arc furnace consists of a circular-shaped vessel with a removable roof, through which project three carbon electrodes, which can be raised or lowered. The process consists of withdrawing the electrodes and swinging open the roof, after which steel scrap/DRI charge is deposited inside the vessel. The roof is swung back into place and the electrodes are lowered into the furnace. A very high electric current is passed through the scrap and an arc is struck with the electrodes, which causes the charge to melt. Thereafter, lime, dolomite and fluorspar are added in order to scavenge out the impurities such as sulphur and phosphorus, to form a slag on top of the molten metal. Samples are taken to check the composition of the material and various ferroalloys are added to adjust the chemistry of the steel. When the required composition of the metal is reached the temperature is controlled in order to achieve the correct temperature for casting. After this the slag is poured off, the furnace is tilted and the steel is poured off into a teeming ladle, which is then carried away and poured into moulds to form ingots. Alternatively, the molten steel can go straight to the continuous casting plant so by-passing the ingot stage.
With this method of steel making, high-grade steels can be produced because within the process there is a high degree of control and refinement possible, and also impurities are reduced to a minimum. One other advantage is that the furnace can be operated entirely on steel scrap/DRI charges and steel can be produced without the assistance of a blast furnace.
In recent years, the quality of steel scrap has reduced metallurgically because of higher residual, or tramp elements, and so there has been a trend by more companies to use direct reduced iron (DRI) mixed with the steel scrap charge.
The Cast Product Forms
The molten steel from the BOF or EAF methods can be cast in one of a number of ways. From the BOF process the charge can be cast into ingots or continuous cast into slabs or billet. These are further processed normally by hot rolling/forging type processes. In the “continuous casting” method of steel making, the molten metal is poured from the converter directly into a refractory-lined ladle, referred to as a “teeming ladle”. The teeming ladle is taken to the top of a tower-type construction where the molten metal gravitates down through a cooling chamber and then through rollers that form the molten steel into bars and/or slabs. With this method, the ingot stage is by-passed and savings are...
Table of contents
- Cover Page
- Half Title Page
- Series Page
- Title Page
- Copyright Page
- Foreword
- Preface
- Acknowledgments
- Contents
- List of Figures
- List of Photographs
- 1. Manufacturing of Iron and Steel
- 2. Types of Steel
- 3. Carriage of Steel
- 4. Surveying of Steel
- 5. Handling of Steel Claims
- Appendices
- Index