
eBook - ePub
Reinventing Patient Recruitment
Revolutionary Ideas for Clinical Trial Success
- 276 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Reinventing Patient Recruitment
Revolutionary Ideas for Clinical Trial Success
About this book
During the last five years, clinical research and development costs have risen exponentially without a proportionate increase in the number of new medications. While patient recruitment for clinical studies is only one component in the development of a new medicine or treatment, it is one of the most significant bottlenecks in the overall drug development process. Now it is imperative that industry leaders see beyond reactive measures and recognize that advancing their approach to patient recruitment is absolutely essential to advancing medicine and continuing the stability of their corporate brand across the globe. Reinventing Patient Recruitment: Revolutionary Ideas for Clinical Trial Success is a definitive guide to planning, implementing and evaluating recruitment strategies and campaigns globally. The combined experience of the authors provides a depth of perspective and boldness of innovative leadership to set the standards for future patient recruitment programs and practices. This book is a must-have for pharmaceutical, biotechnology and medical device industry professionals concerned with enrolling for domestic and multinational clinical studies and remaining on time and on budget.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Reinventing Patient Recruitment by Joan F. Bachenheimer,Bonnie A. Brescia in PDF and/or ePUB format, as well as other popular books in Business & Business General. We have over one million books available in our catalogue for you to explore.
Information
SECTION 2
DEVELOPMENT AND IMPLEMENTATION
CHAPTER SIX
THE IMPORTANCE OF PATIENT PROTECTIONS
“ALWAYS DO RIGHT. THIS WILL GRATIFY SOME PEOPLE, AND ASTONISH THE REST.”
IN THIS CHAPTER
➞ Considering patient protections in an historical perspective
➞ Why knowledge of patient protections is critical to improving patient recruitment
➞ Industry’s responsibility for disseminating information about international patient protections
➞ The Nuremberg Code
➞ The Declaration of Helsinki
➞ Council for International Organizations of Medical Sciences (CIOMS) Guidelines
➞ The Belmont Report and the Common Rule
➞ Institutional Review Boards (IRBs)/Ethics Committee Supervision
➞ Informed Consent Process
➞ Good Clinical Practice (GCP) and International Conference on Harmonization Good Clinical Practice (ICH GCP)
➞ European Union Clinical Trials Directive
HISTORICAL PERSPECTIVE
The first order of business for anyone involved in clinical research who wants to look at patient protections is to recognize the role past errors in our industry have played in creating public skepticism and mistrust of studies. Consider just a few examples:
•The Tuskegee Syphilis Study by the US Public Health Service, conducted between 1932 and 1972, involved 600 African-American men who were misled about the nature of the study, were not given the opportunity to withdraw and were denied adequate treatment for their illness.1
•In 1963, researchers at New York’s Jewish Chronic Disease Hospital injected cancer cells into debilitated patients without informing them, in order to study how these cells were rejected.2
•Newly enrolled patients at New York’s Willowbrook State School for “mentally defective” children were deliberately infected with hepatitis during the early 1960s without adequate freedom of consent from parents and guardians.3
•In May 2001, a healthy 24-year-old employee at Johns Hopkins Asthma and Allergy Center inhaled hexamethonium as part of a research study, which led to her death a month later. The subsequent investigation showed her consent document had failed to adequately describe the research procedures, failed to identify those procedures as experimental and inadequately described foreseeable risks and discomforts. The investigation also showed the researchers had failed to follow approved research protocol, neglected to report problems that surfaced in an initial patient and continued to enroll patients before fully resolving the problems.4
In addition, even though the medical experimentation conducted in the Nazi concentration camps was in no way part of any sanctioned industry study, widespread knowledge of these horrors still informs public attitudes toward medical activity perceived as “experimental.” Only by accepting the consequences of all these historical events and through continual improvement in study procedures and communications will we be able to build greater public trust.
PATIENT PROTECTIONS: GET THE WORD OUT
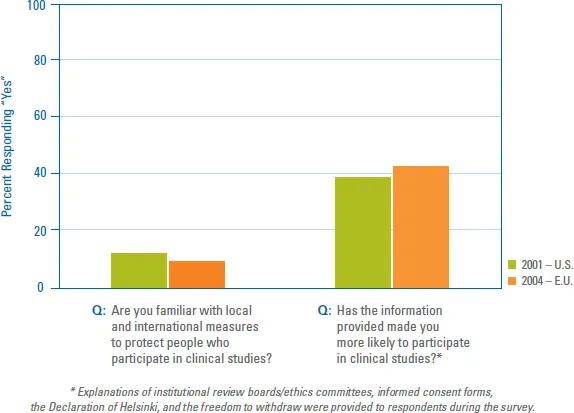
Most sponsors and site staff are aware of the international guidelines protecting patients who participate in clinical trials. What many still do not know is how important it is to actively educate the public about these protections because they can be instrumental in increasing patient participation. BBK’s 2001 “Will & Why Survey” of more than 5,000 people in the US showed that 81 percent were not aware of safeguards like the Declaration of Helsinki, The Belmont Report, Institutional Review Boards (IRBs) and the informed consent process. However, after learning about these protective measures, nearly 40 percent of respondents reported they would be more likely to participate in a research study. In addition, 66 percent indicated they believed that if the general public were aware of these protections, they too would be more willing to participate.
Eighty-one percent of respondents in the US say they are not aware of federal and international measures designed to protect people participating in research studies.i
BBK’s “2004 International Will & Why Survey” showed similar results. Seventy-one percent of more than 2,300 respondents in the Czech Republic, France, Germany, Poland, Spain and the United Kingdom reported they too were not aware of international measures to protect patients in clinical studies. And 42 percent indicated they would be more likely to participate in studies after learning about those protections. Clearly it’s in the best interest of the entire clinical research industry to communicate more extensively about patient protections and to include information about these protections in all patient education materials.
THE NUREMBERG CODE
The Nuremberg Code was created in 1947 after the world became aware of the Nazi medical experiments conducted on people in concentration camps during World War II. It was the first set of principles to outline a code of ethics for medical professionals. The Nuremberg Code first established that experimentation on animals should precede human involvement and that all unnecessary physical and mental suffering and injury should be avoided. Perhaps most importantly, the Nuremberg Code states that participants must always be at liberty to withdraw from experiments. This Code served as the international model for many later standards, all developed to ensure that research with human subjects would be carried out in an ethical manner.
A SUMMARY OF THE 10 PRINCIPLES OF THE NUREMBERG CODE5
1.The voluntary consent of the human subject is essential and must be obtained without coercion.
2.The experiment should be intended to yield fruitful results for the good of society that cannot be obtained in any other way.
3.Experiments with human subjects should be based on results from animal experimentation and knowledge of the history of the disease.
4.Each experiment should be conducted to avoid all unnecessary physical and mental suffering and injury.
5.No experiment may be conducted where there is reason to believe death or disabling injury will occur.
6.The degree of risk undertaken by a human subject should never exceed the humanitarian importance of the experiment.
7.Proper preparations and adequate facilities should be provided to protect the participant from injury, disability, or death.
8.The experiment should be conducted only by scientifically qualified personnel using the highest degree of skill and care.
9.The participant must always be at liberty to leave the experiment.
10.The professional conducting the experiment should be ready to terminate the experiment any time it is determined that continuation may result in injury, disability, or death to a subject.
THE DECLARATION OF HELSINKI
Following dissemination of the Nuremberg Code, the World Medical Association developed the Declaration of Helsinki in 1964 to serve as an ethical guide for physicians conducting medical research on human participants. It was the international medical community’s first significant attempt to regulate itself. It’s been revised five times, most recently in October 2000 and consists of 32 principles. The Declaration...
Table of contents
- Cover
- Half Title
- Title Page
- Copyright Page
- Table of Contents
- Foreword
- Introduction
- Acknowledgements
- About the Authors
- Section One: Getting Started
- Section Two: Development and Implementation
- Section Three: Going Global
- Section Four: Future Trends
- Section Five: Appendices
- Glossary
- Index