![]()
1Iodine products that are deeply rooted in our everyday life
From mouthwash to LCD television
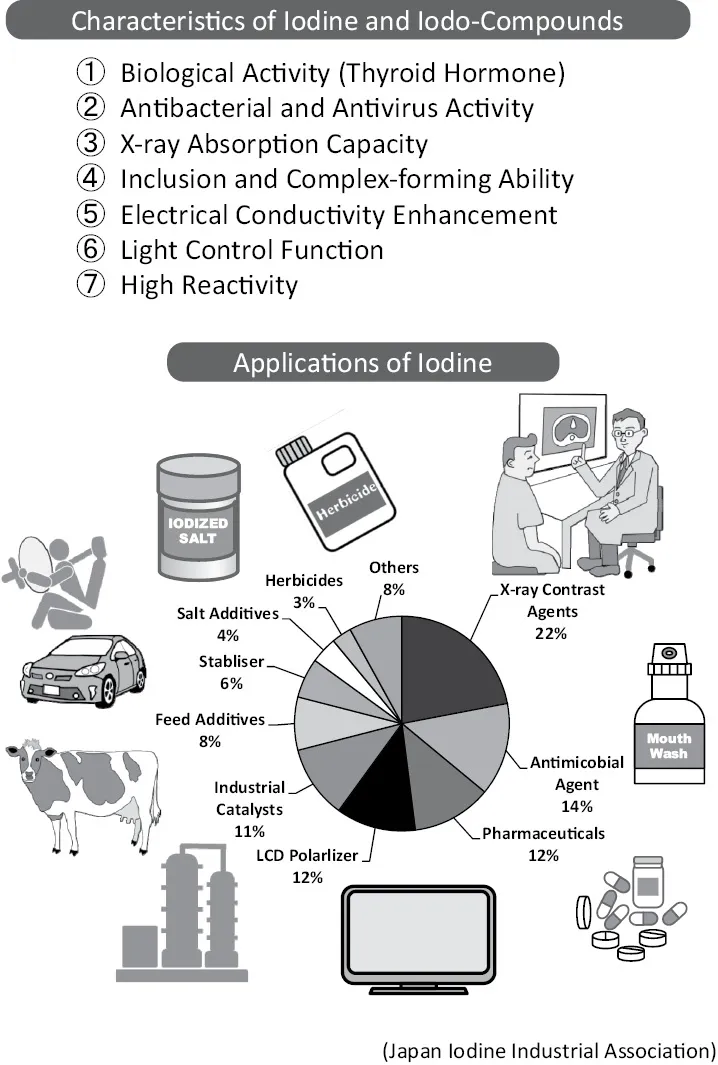
Iodine is deeply rooted in our everyday life. Products containing iodine atoms or molecules demonstrate properties such as “blocks X-rays, shows high reactivity, forms various compounds with most other elements, and provides strong anti-bacterial effect.” These superior functions are seen in many areas in our daily life.
The specific capacity of iodine to absorb X-rays is used in contrast agents for X-rays. It is used in hospitals to create detailed images of the brain and heart. Iodine is also used to control light. It is used widely in LCD (liquid-crystal display) screens for TVs, mobile phones, and car navigation systems. Iodine is used in the polarizing film which acts as the switch on the screens. Depending on the specific direction the polarizing film arranges the iodine, it can filter light.
Industrial catalysts take advantage of the high reactivity of iodine. Acetic acid is produced from methanol and carbon monoxide as raw materials. This reaction is catalyzed by the metal (iridium or rhodium) complex with iodine and proceeds under mild conditions.
In automobile tires and airbags, nylon wire is used for reinforcement. To prevent oxidization or deterioration under high temperatures, potassium iodide or copper iodide is added as a stabilizer.
There is a recently developed insecticide in Japan called Flubendiamide, which combines fluorine with iodine. This chemical takes advantage of various halogen properties. Iodine molecules are high in antibacterial properties and have been used in a tincture of iodine for many years. Recently, various stabilized chemicals with a combination of polymers have appeared, a typical example being povidone iodine (Betadine).
Iodine is an essential element for living creatures. Iodine deficiency is caused by a lack of iodine intake. In Western countries, potassium iodate is added to table salt. Iodine is also indispensable for livestock and is provided as a feed additive [1].
Summary Box
•Without iodine, X-ray contrast agents and LCD polarizing films cannot be produced.
•Without iodine, humans and animals cannot live.
2 Iodine reacts with most elements
It is the heaviest and largest of all stable halogen elements
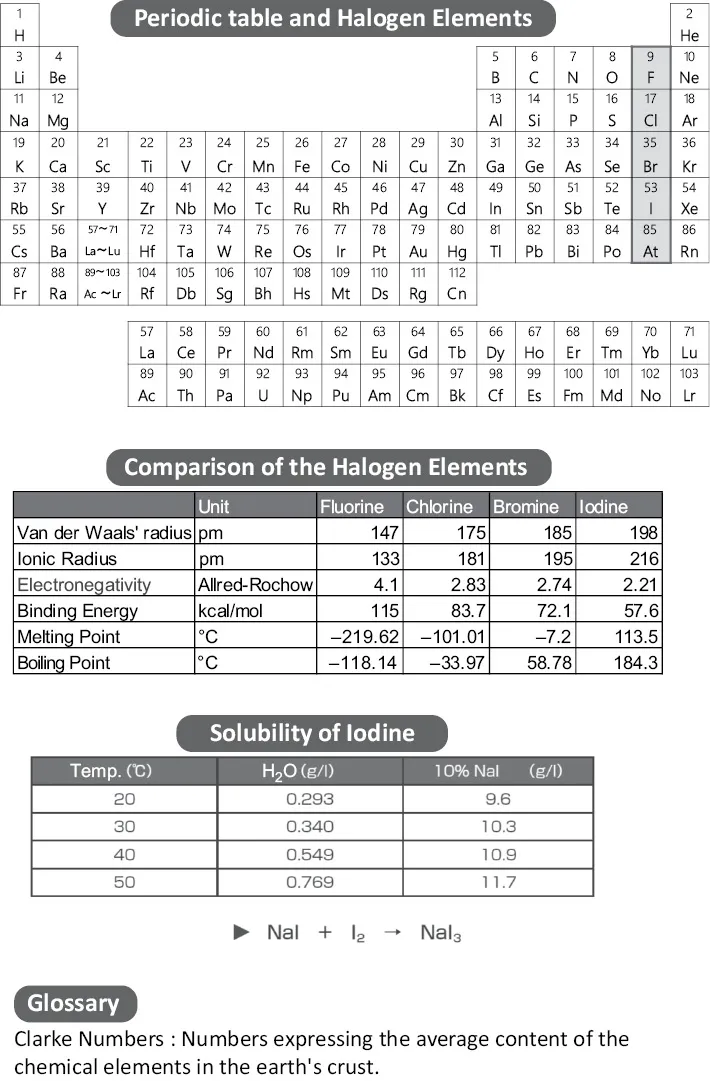
Iodine is one of the five halogen elements listed on the periodic table, and is located next to the noble gases. Of the stable halogens (fluorine, chlorine, bromine, iodine), excluding astatine which is radioactive, iodine is the largest element. A comparison of iodine with other halogen elements is shown in the chart, including van der Waals’ radius which indicates the size of the atom, electronegativity which indicates the tendency of an atom to attract a bonding pair of electrons, and the binding energy for carbon. At room temperature, fluorine and chlorine are gas, and bromine is liquid. In contrast, iodine is a solid and has a melting point of 113.5°C.
The specific gravity of iodine is 4.93, which is heavy compared to metals (iron 7.85, titanium 4.61). Similar to camphor and naphthalene, iodine can sublimate, in other words change directly from solid to gas (without passing through the intermediate liquid phase). If iodine is placed in a jar, part of it change to a purple gas. Clarke number for iodine is 0.5, and is positioned as the 64th among the elements and is a vital resource. Iodine reacts to most elements excluding noble gases, and forms iodides. These properties of iodine are used in the recovery of rare metals.
Iodine is highly soluble in organic solvents. Depending on the characteristics of the solvent, solutions of varying color such as purple, red, and brown can be created. For example, chloroform and hexane solutions are purple, benzene, toluene, xylene solutions are red, and methanol, ethanol, and acetic acid solutions are brown. On the other hand, iodine does not dissolve well in water. However, in sodium iodide or potassium iodide solutions, the reaction shown in the bottom diagram occurs, producing polyiodide ion (I3−, I5−, etc.), which becomes easily soluble.
As described above, among the halogen elements, iodine is very unique [2].
Summary Box
•Iodine is a solid halogen element at room temperature with sublimabilty.
•Iodine has properties similar to metal.
3 Iodine was discovered in France
Bernard Courtois, the chemist who discovered iodine
From ancient times, seaweed ash has been used as an ingredient for glass and fertilizers in the Brittany and Normandy regions of northwest France. In particular, during the Napoleonic Wars in the early nineteenth century, saltpeter (potassium nitrate, KNO3), a component of gunpowder, was widely manufactured from seaweed, which is a souse of potassium. When manufacturing saltpeter, Bernard Courtois, a chemist, added too much sulfuric acid to seaweed ash, causing the iodine to evaporate and creating iodine crystals. To this day, the historic ruins of a stone seaweed incinerator can be seen in Brittany. Furthermore a monument to the discovery of iodine and buildings of the old iodine factory can be seen near the coast.
It was in 1811 that Courtois obtained those iodine crystals. Courtois, with the help of two friends (Charles-Bernard Desormes and Nicolas Clément) and Joseph Louis Gay-Lussac, verified iodine to be a new element and published an article in a French journal (Annales de Chimie) in December, 1813 [3a].
The Napoleonic Wars ended in 1815, and in 1820, the pharmaco...