
Critical Appraisal from Papers to Patient
A Practical Guide
- 201 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Critical Appraisal from Papers to Patient
A Practical Guide
About this book
Having an understanding of critical appraisal and evidence-based medicine is a prerequisite to being an effective clinician. However, critical appraisal is often taught by people not involved in day-to-day clinical care, meaning the clinical relevance is not always brought to the fore.
This book takes a different approach. It is written by clinicians, for clinicians. It takes the reader step by step through the process so that any journal article can be easily read, evidence evaluated and the results - and their reliability - truly understood. By integrating this with knowledge of local skills and resources and patient preference, the reader will be able to apply the best possible care that his or her patients deserve.
This accessible book is suitable for undergraduates and postgraduates of all medical specialties, nursing, paramedics, pharmacists and all allied health professions. It is the ideal reference for anyone needing help writing a clinical topic review, reviewing a paper in a journal club or preparing for a critical appraisal exam. But most importantly, it is essential for those who wish to practice medicine in the best possible way for their patients, using the best evidence, tailored to the individual.
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
Section 1
Introduction to Critical Appraisal
1 Types of Papers and Grades of Evidence
TYPES OF PAPERS
- What sort of environment (a clinical laboratory, a highly controlled but still clinical setting, a normal ward or general practitioner’s surgery, etc.) is the trial being performed in?
- What sort of results are the researchers interested in? Is it a result like mortality rate or blood pressure which will provide a numerical value, or is it a subjective feeling or description of outcomes and emotions which cannot be reduced to a numerical value (qualitative research)?
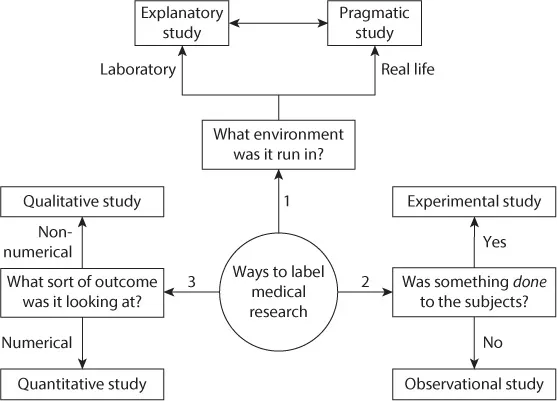 FIGURE 1.1 Different ways to label a trial – three questions to ask.
FIGURE 1.1 Different ways to label a trial – three questions to ask. - Are the researchers planning to do something to the patient, for example, give them a new drug or do a new diagnostic test, or are they just observing what happens to them?
QUESTION 1: WHAT SORT OF ENVIRONMENT WAS THE TRIAL RUN IN?
QUESTION 2: WHAT SORT OF RESULTS ARE THE RESEARCHERS INTERESTED IN?
QUESTION 3: ARE THE RESEARCHERS PLANNING TO DO SOMETHING TO THE PATIENT?
Different Types of Experimental Studies
Randomized controlled study (RCT) | The study group is divided into two or more groups by a randomization process. One group is given one intervention, and the other group(s) another. The outcomes in the different groups are then compared. The randomization works to balance and thereby remove the influence of confounding factors. The RCT is considered to be the best sort of experimental study and commonly used in many examinations. They are, however, normally expensive to run. |
Diagnostic study | The diagnostic test is performed on the whole study group alongside the gold standard test. The performance of the diagnostic test under investigation is then compared with the gold standard test. Diagnostic studies appear less commonly than RCTs in the literature but appear commonly in many examinations. |
Crossover study | The study is designed to compare two interventions (usually drugs). Instead of one group receiving one drug and the other group the second drug, both groups receive one drug for a period of time, followed by the alternate drug for a period of time. The difference between the two groups is the orde... |
Table of contents
- Cover
- Title Page
- Copyright Page
- Contents
- Foreword
- Authors
- Introduction
- Section 1 Introduction to Critical Appraisal
- Section 2 Basic Statistics
- Section 3 Appraising Different Types of Papers
- Section 4 Applying the Knowledge
- Section 5 Critical Appraisal Exams
- Glossary
- Index