
- 211 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
About this book
Biochemistry Explained employs an innovative approach which has proven highly successful in the author's own classes. The author establishes a thorough understanding of the foundations of and common linkages between molecular structures and reactions, so that eventual interpretation of complex biochemical pathways and reactions is easy. All of the major molecular structures and biochemical pathways are explained, and, for the most part, these center on mammalian biochemistry. The text is supported by biochemical nomenclature and questions to bear in mind while reading. Higher learning sections are also provided for advanced students. Written in an informal, conversational style, this textbook will serve as an invaluable resource for any student who is struggling with the standard texts and for postgraduate students who need to refresh their knowledge.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
1 Fundamental Concepts and an Introduction to the Cell
- be introduced to the idea that lipid and water solubility is a major foundation stone of biochemistry
- learn which chemical structures determine water and lipid solubility
- be introduced to the terms hydrophobic and hydrophilic
- revise subcellular compartments and their functions
- learn the function of the cytoskeleton.
Lipid and water solubility
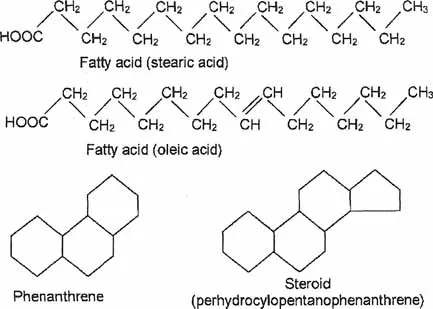
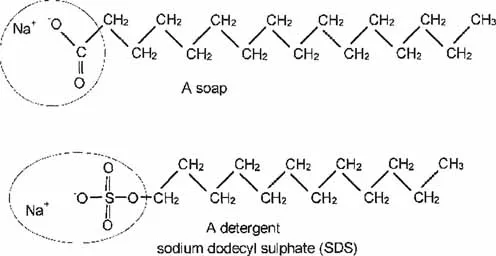
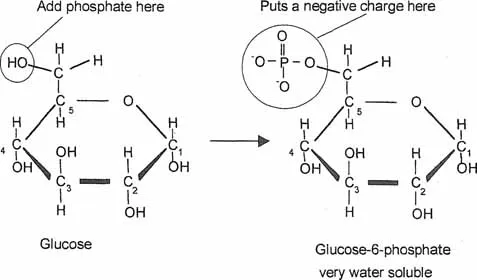
The structure of a cell
Table of contents
- Cover
- Half Title
- Title Page
- Copyright Page
- Table of Contents
- Dedication Page
- Preface
- For the Lecturer
- Chapter 1 Fundamental Concepts and an Introduction to the Cell
- Chapter 2 Basic Biochemicdl Structures
- Chapter 3 Sugars
- Chapter 4 Amino Acids and their Functions
- Chapter 5 Peptides, Proteins and Enzymes
- Chapter 6 Complex Sugars and their Functions
- Chapter 7 Metabolism of Sugars
- Chapter 8 Metabolism of Amino Acids
- Chapter 9 The Tricarboxylic Acid Cycle
- Chapter 10 The Electron Transport Chain, Oxidative Phosphorylation and Photosynthesis
- Chapter 11 Lipid Structure and Metabolism
- Chapter 12 Nucleotides and their Derivatives
- Chapter 13 The Structures and Functions of DNA and RNA
- Chapter 14 Metabolism and its Control
- Chapter 15 Cellular Communication
- Index
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app