
- 875 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Ceramic Processing and Sintering
About this book
As the field's premiere source, this reference is extensively revised and expanded to collect hard-to-find applications, equations, derivations, and examples illustrating the latest developments in ceramic processing technology. This book is concerned primarily with the processing of polycrystalline ceramics and focuses on the widespread fabrication of ceramics by the firing of consolidated powders forms. A brief treatment of sol-gel processing is also included.
Ceramic Processing and Sintering, Second Edition provides clear and intensive discussions on colloidal and sol-gel processing, sintering of ceramics, and kinetic processes in materials. From powder synthesis and consolidation to sintering and densification behavior, this latest edition emphasizes the impact of each processing procedure on ceramic properties. The second edition also contains new and extended discussions on colloid stability, polymer growth and gelation, additives in ceramic forming, diffusion and defect strucutre, normal and abnormal grain growth, microwave sintering, Rayleigh instability effects, and Ostwald ripening.
Illustrating the interconnectedness between the various steps in the overall fabrication route, Ceramic Processing and Sintering, Second Edition approaches the fundamental issues of each process and show how they are applied to the practical fabrication of ceramics.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
1
Ceramic Fabrication Processes
1.1 Introduction
The subject of ceramics covers a wide range of materials. Recent attempts have been made to divide it into two parts: traditional ceramics and advanced ceramics. The use of the term advanced has, however, not received general acceptance and other forms including technical, special, fine, and engineering will also be encountered. Traditional ceramics bear a close relationship to those materials that have been developed since the earliest civilizations. They are pottery, structural clay products, and clay-based refractories, with which we may also group cements and concretes and glasses. Whereas traditional ceramics still represent a major part of the ceramics industry, the interest in recent years has focused on advanced ceramics, ceramics that with minor exceptions have been developed within the last 50 years or so. Advanced ceramics include ceramics for electrical, magnetic, electronic, and optical applications (sometimes referred to as functional ceramics) and ceramics for structural applications at ambient as well as at elevated temperatures (structural ceramics). Although the distinction between traditional and advanced ceramics may be referred to in this book occasionally for convenience, we do not wish to overemphasize it. There is much to be gained through continued interaction between the traditional and the advanced sectors.
Chemically, with the exception of carbon, ceramics are nonmetallic, inorganic compounds. Examples are the silicates such as kaolinite [Al2Si2O5(OH)4] and mullite (Al6Si2O13), simple oxides such as alumina (Al2O3) and zirconia (ZrO2), complex oxides other than the silicates such as barium titanate (BaTiO3), and the superconducting material YBa2Cu3O6+δ (0 ≤ δ ≤ 1). In addition, there are nonoxides including carbides such as silicon carbide (SiC) and boron carbide (B4C), nitrides such as silicon nitride (Si3N4) and boron nitride (BN), borides such titanium diboride (TiB2), silicides such as molybdenum disilicide (MoSi2) and halides such as lithium fluoride (LiF). There are also compounds based on nitride–oxide or oxynitride systems (e.g., β’-sialons with the general formula Si6-zAlzN8-zOz, where 0 < z < ~4).
Structurally, all materials are either crystalline or amorphous (also referred to as glassy). The difficulty and expense of growing single crystals means that, normally, crystalline ceramics (and metals) are actually polycrystalline—they are made up of a large number of small crystals, or grains, separated from one another by grain boundaries. In ceramics as well as in metals, we are concerned with two types of structure, both of which have a profound effect on properties. The first type of structure is at the atomic scale: the type of bonding and the crystal structure (for a crystalline ceramic) or the amorphous structure (if it is glassy). The second type of structure is at a larger scale: the microstructure, which refers to the nature, quantity, and distribution of the structural elements or phases in the ceramic (e.g., crystals, glass, and porosity).
It is sometimes useful to distinguish between the intrinsic properties of a material and the properties that depend on the microstructure. The intrinsic properties are determined by the structure at the atomic scale and are properties that are not susceptible to significant change by modification of the microstructure, properties such as the melting point, elastic modulus, coefficient of thermal expansion, and whether the material is brittle, magnetic, ferroelectric, or semiconducting. In contrast, many of the properties critical to the engineering applications of materials are strongly dependent on the microstructure (e.g., mechanical strength, dielectric constant, and electrical conductivity).
Intrinsically, ceramics usually have high melting points and are therefore generally described as refractory. They are also usually hard, brittle, and chemically inert. This chemical inertness is usually taken for granted, for example, in ceramic and glass tableware and in the bricks, mortar, and glass of our houses. However, when used at high temperatures, as in the chemical and metallurgical industries, this chemical inertness is severely tried. The electrical, magnetic, and dielectric behavior covers a wide range—for example, in the case of electrical behavior, from insulators to conductors.
The applications of ceramics are many. Usually, for a given application one property may be of particular importance, but in fact, all relevant properties need to be considered. We are therefore usually interested in combinations of properties. For traditional ceramics and glasses, familiar applications include structural building materials (e.g., bricks and roofing tile), refractories for furnace linings, tableware and sanitaryware, electrical insulation (e.g., electrical porcelain and steatite), glass containers, and glasses for building and transportation vehicles. The applications for which advanced ceramics have been developed or proposed are already very diverse and this area is expected to continue to grow at a reasonable rate. Table 1.1 illustrates some of the applications for advanced ceramics (1).
The important relationships between chemical composition, atomic structure, fabrication, microstructure, and properties of polycrystalline ceramics are illustrated in Fig. 1.1. The intrinsic properties must be considered at the time of materials selection. For example, the phenomenon of ferroelectricity originates in the perovskite crystal structure, of which BaTiO3 is a good example. For the production of a ferroelectric material, we may therefore wish to select BaTiO3. The role of the fabrication process, then, is to produce microstructures with the desired engineering properties. For example, the measured dielectric constant of the fabricated BaTiO3 will depend significantly on the microstructure (grain size, porosity, and presence of any secondary phases). Normally, the overall fabrication method can be divided into a few or several discrete steps, depending on the complexity of the method. Although there is no generally accepted terminology, we will refer to these discrete steps as processing steps. The fabrication of a ceramic body therefore involves a number of processing steps. In the next section, we examine, in general terms, some of the commonly used methods for the fabrication of ceramics.
1.2 Ceramic Fabrication Processes
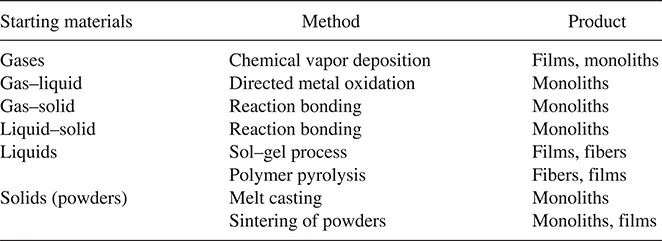
Ceramics can be fabricated by a variety of methods, some of which have their origins in early civilization. Our normal objective is the production, from suitable starting materials, of a solid product with the desired shape such as a film, fiber, or monolith and with the desired microstructure. As a first attempt, we divide the main fabrication methods into three groups, depending on whether the starting materials involve a gaseous phase, a liquid phase, or a solid phase (Table 1.2). In the following sections, we examine briefly the main features of the processing steps involved in these methods and, from the point of view of ease of processing, their main advantages and disadvantages.
1.2.1 Gas-Phase Reactions
By far the most important are vapor deposition methods, where the desired material is formed by chemical reaction between gaseous species. The reaction between a liquid and a gas is generally impractical but has been developed recently into an elegant technique, referred to as directed metal oxidation. Reaction between a gas and a solid, commonly referred to as reaction bonding (or reaction forming) has been used mainly for the production of Si3N4 but is now also being applied to the production of oxide ceramics. Reaction bonding (by a solid–liquid reaction) is also an important fabrication route for SiC.
TABLE 1.1 Application of Advanced Ceramics Classified by Function

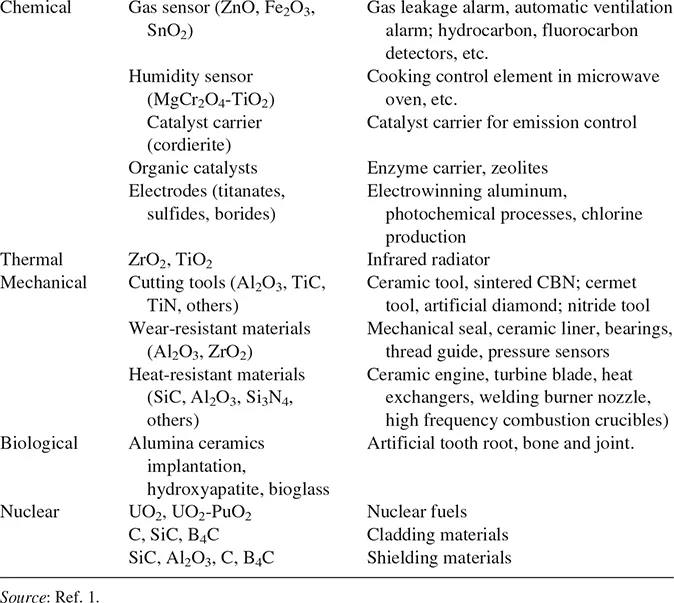
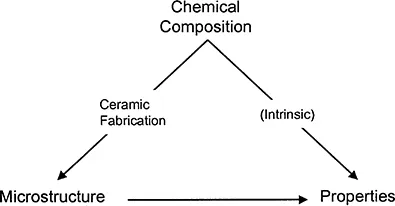
FIGURE 1.1 The important relationships in ceramic fabrication.
TABLE 1.2 Common Ceramic Fabrication Methods
1.2.1.1 Chemical Vapor Deposition
Chemical vapor deposition (CVD) is a process by which reactive molecules in the gas phase are transported to a surface at which they chemically react and form a solid film. It is a well-established technique that can be used to deposit all classes of materials, including metals, ceramics, and semiconductors, for a variety of applications. Large areas can be coated and the process is amenable to mass production. Thick films or even monolithic bodies can also be produced by basically prolonging the deposition process so that the desired thickness is achieved. Table 1.3 shows some of the important reactions used for the fabrication of ceramics toge...
Table of contents
- Cover
- Half Title
- Title Page
- Copyright Page
- Dedication
- Table of Contents
- Preface to the Second Edition
- Preface to the First Edition
- 1 Ceramic Fabrication Processes: An Introductory Overview
- 2 Synthesis of Powders
- 3 Powder Characterization
- 4 Science of Colloidal Processing
- 5 Sol–Gel Processing
- 6 Powder Consolidation and Forming of Ceramics
- 7 Sintering of Ceramics: Fundamentals
- 8 Theory of Solid-State and Viscous Sintering
- 9 Grain Growth and Microstructure Control
- 10 Liquid-Phase Sintering
- 11 Special Topics in Sintering
- 12 Densification Process Variables and Densification Practice
- Appendix A: Physical Constants
- Appendix B: SI Units—Names and Symbols
- Appendix C: Conversion of Units and Decimal Fractions and Multiples
- Appendix D: Ionic Crystal Radii (in units of 10–10 m)
- Appendix E: Density and Melting Point of Some Elements, Ceramics, and Minerals
- Appendix F: Aperture Size of U.S. Standard Wire Mesh Sieves (ASTM E 11:87)
- Index
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Ceramic Processing and Sintering by Mohamed N. Rahaman in PDF and/or ePUB format, as well as other popular books in Technology & Engineering & Industrial & Technical Chemistry. We have over one million books available in our catalogue for you to explore.