
- 320 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Introduction to Nuclear Power
About this book
The authors of this text aim to educate the reader on nuclear power and its future potential. It focuses on nuclear accidents such as Chernobyl and Three Mile Island, and their consequences, with the understanding that there are safety lessons to be learned if nuclear power generation is going to be expanded to meet our growing energy needs.
Information
1
The Earth and Nuclear Power
Sources and Resources
1.1 INTRODUCTION
This book is written from an engineer’s viewpoint, particularly that of a thermal engineer, that is, a design or research engineer concerned with heat production and utilization. We believe that the most important problems in the utilization of nuclear power concern the handling of thermal energy generated in the various processes. This includes handling under the normal operating and processing conditions and dealing with heat removal problems under the unlikely conditions of an accident. The problem of handling thermal energy associated with nuclear power does not stop when the fuel is removed from the power station; small amounts of heat are generated in the spent fuel before it is processed and in the waste products. The consequences of this are also the concern of the thermal engineer.
The approach that we shall take, therefore, is one that is not normally followed in general books on nuclear energy. We will follow the history of nuclear materials from their cosmic origins, through their terrestrial life span up to the time when they are used in nuclear reactors, and beyond. Although we will need to explain some elementary aspects of physics, the emphasis will be on what happens to the thermal energy.
We begin with the history of uranium in the earth, the decay of its isotopes, and the effect this decay has had on the earth as we know it. Comparisons are made with the earth’s other main energy source: the sun. Energy from the sun is derived either directly or through storage media such as fossil fuels, hydroelectric power, and winds.
The rate at which energy may be extracted from nuclear materials can be enhanced by the self-sustaining process of nuclear fission. Nuclear fission does not normally occur in nature, but recent studies have revealed that nature anticipated Enrico Fermi by about 2 billion years in creating a natural nuclear fission reactor by a series of extraordinary and improbable events. We shall use this example in introducing nuclear fission.
In the final part of this chapter, we compare the relative magnitudes of thermal energy resources of the various types: fossil fuel, nuclear, solar, and so forth.
1.1.1 Forms of Energy
What is energy? There is general awareness of the problem of depletion of the world’s energy resources. People understand energy in terms of those resources, namely, the supplies of oil, gas, and coal and the electricity derived from them. All of these items have made an increasingly large demand on national and personal budgets.
The engineer has, by training, a somewhat different concept of energy. This derives from his or her undergraduate training in the field of thermodynamics, which is the science of energy and energy conversion. We do not intend to try to provide a basic course in thermodynamics; however, for the rest of this book to be reasonably intelligible, it is important that some of the basic concepts be stated.
The concept of doing work to lift objects or to move an object such as a bicycle along is a commonly accepted one. Thus, it is relatively easy to understand the concept of energy as a measure of the ability to do work. Energy can appear in different forms as follows:
1. Kinetic Energy. This is energy associated with movement, for example, that of a flywheel or a moving locomotive.
2. Potential Energy. This is energy possessed by virtue of position, typically in the earth’s gravitational field. For instance, a child sitting on the higher end of a seesaw has greater potential energy than a child sitting on the lower end. Likewise, water in a mountain lake has greater potential energy than water at sea level.
3. Chemical Energy. Matter consists of atoms that are combined together in molecules. Molecules of different substances can react to release energy, and this releasable energy is often termed chemical energy. For example, chemical energy is released when gasoline combines with air in the cylinders of a car’s engine.
4. Electrical Energy. Atoms consist of a central mass, known as the nucleus, around which a cloud of electrons circulates (see Figure 1.1). If there is an excess or deficit of electrons in one part of a body, the body is said to have an electrical charge and, by virtue of this, to have electrical energy. An example of this is a thunderstorm, where the clouds are charged electrically with respect to the ground.
5. Nuclear Energy. Normally, the nucleus of an atom is stable and will remain indefinitely in its present state. An example is the nucleus of an atom of iron; no matter how much we would like it to happen, iron will never change into another element, such as gold. However, the atoms of some elements are unstable and can change into another form spontaneously, by the emission of radiation. We shall discuss the forms of radiation emitted further in Section 1.2; it is sufficient here to note that the radiation emitted has kinetic energy and the disintegration process results in the release of energy associated with the nucleus, namely, the nuclear energy. If the nucleus could be weighed before the disintegration, and the resulting nucleus and all particulate components of the radiation weighed afterward, it would be observed that a small change in mass had occurred due to the conversion of mass into energy. The relationship between the loss of mass m3 and the energy released E is given by Einstein’s famous equation:
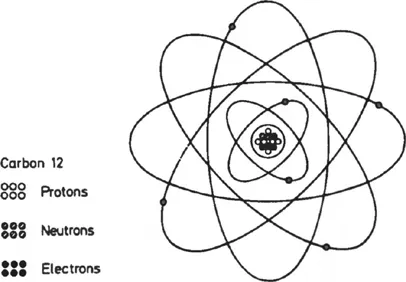
Figure 1.1: Schematic diagram of carbon-12 atom.

where c is the velocity of light, namely 300,000 kilometers per second (186,000 miles per second). The amount of energy deriving from a mass loss is enormous; for example, 100 kilograms of mass fully converted into energy would supply all the energy needs of the United Kingdom (at the present rate of usage) for a year. Each kilogram of mass, fully converted, is equivalent to the energy available by burning 3 million tons of coal. In a typical nuclear reaction, however, only a tiny fraction of the mass is converted into energy, typically ~0. 1%. The disintegration of an unstable nucleus, and the consequent release of nuclear energy, can be stimulated by exciting the nucleus by bombarding it with radiation. This is at the heart of the fission reaction process, which we shall discuss further below. Nuclear energy can also be released, as we shall see, by the fusion of very light atoms into heavier ones.
6. Thermal Energy. The atoms of all substances are in constant motion. In a solid the atoms are held in an approximately fixed position with respect to one another. However, they all vibrate to an extent that increases with increasing temperature. The energy associated with this vibration is called thermal energy. In fluids (namely, liquids and gases), two or more atoms may be combined with each other chemically in the form of molecules. These molecules have vibrational energy, but in the fluid state they may also have translational energy arising from their motion in space and rotational energy arising from their rotation. All of these components of energy add up to the thermal energy of the fluid. It will be seen from this description that thermal energy is of a special type. It is associated with atomic or molecular movements that are randomly directed. This makes it very much more difficult to convert thermal energy into other forms of energy, as we shall see below.
The intensity of atomic or molecular movement is a measure of the energy content of a piece of matter. A body that has a high intensity of atomic or molecular movement will transfer energy to an adjacent body with a lower intensity of movement. This process of transfer of thermal energy is known as conduction, and we define a quantity known as temperature as a measure of the ability of a body to transfer thermal energy to adjacent bodies by the conduction process. If the temperature of a body is higher than that of adjacent bodies, heat will be conducted from it; if it is lower, the reverse is true. We conveniently choose a scale of temperature in terms of certain transitions that occur in nature. Specifically, we define the melting point of ice as zero degrees centigrade (0°C) and the boiling point of water as 100 degrees c...
Table of contents
- Cover
- Half Title
- Title Page
- Copyright Page
- Table of Contents
- Preface to Second Edition
- Preface to First Edition
- 1 The Earth and Nuclear Power: Sources and Resources
- 2 How Reactors Work
- 3 Cooling Reactors
- 4 Loss of Cooling
- 5 Loss-of-Cooling Accidents: Some Examples
- 6 Postulated Severe Accidents
- 7 Cooling during Fuel Removal and Processing
- 8 Cooling and Disposing of the Waste
- 9 Fusion Energy: Prospect for the Future
- Index
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Introduction to Nuclear Power by Geoffrey F. Hewitt,John G. Collier in PDF and/or ePUB format, as well as other popular books in Ciencias físicas & Energía. We have over one million books available in our catalogue for you to explore.