![]()
Part I
Overview of Platform Clinical Trials
![]()
1
I-SPY2
Unlocking the Potential of the Platform Trial
Laura Esserman, Nola Hylton, Smita Asare, Christina Yau, Doug Yee, Angie DeMichele, Jane Perlmutter, Fraser Symmans, Laura van’t Veer, Jeff Matthews, Donald A. Berry, and Anna Barker
Modern drug discovery, fueled by high throughput technologies, combinatorial chemistry, rational drug design, and increasingly powerful information processing, has become progressively efficient and productive. So much so, that the number of new cancer therapeutics in development doubled over the span of a decade.1 As of 2015, there were 836 individual cancer drugs at various stages in the pipelines of America’s biopharmaceutical companies, including 83 for breast cancer and 132 for lung.2 An estimated 80% of these are first-in-class therapies and 73% are potential “personalized” medicines.3 On paper at least, we have an embarrassment of riches. But it is a different story in practice.
On average, it takes 10–15 years for a new therapeutic to successfully navigate preclinical and clinical testing and gain marketing approval. Rising costs, particularly for late stage clinical trials, put the average investment required for a new agent past the US$2 billion mark.4
Not only is new drug development a high stakes game, it is also high risk, particularly in oncology: fewer than 1 in 20 agents entering clinical trials ultimately gain approval.5 Two-thirds of agents evaluated in large, expensive phase 3 trials fail to receive approval. The requirement for large patient numbers to achieve statistical certainty in our current trial model compounds our problems. As we prepare for the age of personalized medicine, we must also prepare for the inevitable subdividing of diseases, particularly cancers, into smaller and smaller subsets, making it harder and harder to achieve target enrollment.
Few would argue that our current approach of using successively larger, single agent randomized controlled trials (RCTs) is efficient, in terms of time, money, and resources; it has resulted in inordinately large investments with low yields. Perhaps more importantly, it continues to struggle to meet the needs of patients, who are increasingly demanding better, more personalized treatments.
Faster “Knowledge Turns”
This need for “something better” is what has fueled the principal mission of the I-SPY program: to accelerate the development of improved treatments for early breast cancers at high risk of recurrence. In practice, this has meant nothing less than developing a platform trial model that was smaller, more targeted, faster, more efficient, cheaper, and demonstrating that it could be successfully put into practice. However, from the beginning, I-SPY was designed to be much more.
The overarching goal has been not simply to demonstrate a new trial model in a single indication, but to re-engineer the clinical trial in a manner broadly applicable in oncology and beyond. Conceptually, the goal is to move away from discrete, single agent trials, towards a continuously updated “learning” platform that employs biological information (biomarkers) to progressively optimize the targeting of agents to the patients or subtypes in which they are most effective.
This accelerated learning approach is rooted in the concepts described by former CEO and co-founder of Intel, Andy Grove, PhD who, in a 2005 article in JAMA, compared development cycles between healthcare and the semiconductor industry.6 The rate of progress in the latter is often represented by “Moore’s Law,” which states that the number of components per integrated circuit (and therefore the processor power) doubles every two years.7 This rapid rate of progress, Grove argues, relies on rapid “knowledge turns” and early indicators of success or failure during the development process for a new or improved product. In his industry, knowledge turns are measured in months.
By the same measure, a knowledge turn in healthcare (including oncology), is essentially the transit time to move from a proposed treatment to the analysis of trial results. In drug development, knowledge turns are measured in years—in many indications, even decades. One can point to any number of factors contributing to the extended development times in clinical drug development.
Protocol Approvals
Most investigators are all too aware of the complex processes and multiple approvals required to initiate a single trial—that is, each and every trial.
This redundancy is one of the most significant sources of inefficiency in our system—every principal investigator, company, or cooperative group needs to run their own trial, and for every agent evaluated, a brand-new protocol is written, requiring multiple repetitions of the reviews and regulatory approvals, contracting, and start up time. Then, to make matters worse, at the end of the trial, all the contracts, documentation, and approvals are essentially voided, forcing us to start over on the next (often very similar) trial. This kind of waste of human and intellectual capital would be unacceptable in any other industry, but for clinical trials, it has been the norm for decades. Only recently, with the introduction of the master protocol, have we begun to explore more expedient means of getting to the same place.
Smarter Outcomes
All it takes is a quick glance at the typical timeline for oncology trials and it becomes clear that the most significant contributor to the long knowledge turns in drug development are in follow-up—a consequence of the distant outcome measures that are required. Take for instance, the use of disease-free survival (DFS) as an endpoint in either neoadjuvant or adjuvant therapy trials in oncology. This alone condemns this industry to progress at a snail’s pace, as it establishes our baseline for obtaining results as 5–10 years—this is the lion’s share of the healthcare knowledge turn. Although there is little question DFS remains the gold standard of efficacy, it is important to recognize that perfection (in terms of endpoints) clearly comes at a cost of excruciatingly slow development times. For this reason, validating an early surrogate outcome measure was one of the mission-critical objectives of I-SPY.
As we have learned in I-SPY, the neoadjuvant approach has important advantages over adjuvant therapy in this regard. By starting with systemic therapy as the first treatment (rather than surgical removal), it provides an opportunity to directly assess tumor response to therapy. It allows us to maximize what we learn about the effects of the therapy and to do so very early after treatment, rather than 5–10 years down the road. This has clear advantages not only for the purposes of the trial, but for optimizing care and redirecting treatment to clinical trials or other interventions in the event of a poor response.
Smarter Approach
Another key contributor to the extended development times is that all agents are first tested in the metastatic stage, not only for phase 1 (safety), but for phase 2 and 3, prior to being tested in the early disease phase. Testing novel agents earlier in the disease course (early stage) in the setting of high risk for early recurrence can cut years off the development time. As well, this means testing agents when the disease is more curable, before patients have had multiple treatments and the possibility of developing resistance, and while the immune system is more intact; all contribute to an increased likelihood to improve the chance of a meaningful response, while improving the knowledge turns.
Of course, unlike processor chips, in which great pains are taken to ensure they are identical in both construction and operation, the nature of diseases like cancer couldn’t be more different. Cancers are heterogeneous in multiple ways, they are dynamic, and they adapt to external stimuli. Because this heterogeneity cannot be ignored, I-SPY was designed to embrace it. Biomarkers can fuel adaptive designs to help determine how an agent performs among different molecular subtypes of breast cancer,8 ultimately speeding the transition of drugs from phase 2 to phase 3 trials, and increasing the chances of success in phase 3 by using a more targeted approach.
I-SPY2: Design
The I-SPY Program goals were, from the beginning (and they remain) lofty. It was designed to integrate and link phase 1 (I-SPY Phase 1), phase 2 (I-SPY2), and, in time, Phase 3 (I-SPY3) evaluations to establish a dynamic pipeline of novel agents. We aim to accelerate the identification of the subset of high risk breast cancer patients that benefit from novel agents or combinations, rapidly validate them, and shepherd them into clinical use (Table 1.1). The centerpiece of the program is I-SPY2, our ongoing, adaptively randomized phase 2 study that features multiple simultaneous experimental arms with a common control and a master-protocol. 9, 10
TABLE 1.1
I-SPY2 is designed to increase the success rate of phase 3 trials, understand which subset of disease has the biggest benefit for specific agents and reduce the time and cost of running phase 3 trials.
| Principle | Solution |
| Test agents where they matter most | - Neoadjuvant setting, poor prognosis cancers
- Integrate advocates into trial planning
|
| Rapidly learn to tailor agents | - Adaptive Design
- Neoadjuvant therapy
- Integration of biomarkers, imaging
|
| Optimize Phase 3 trials | - Graduate drugs with predicted probability of success in Phase 3 trials for given biomarker profile
|
| Drive Organizational Efficiency | - Adaptive Design
- Master IND & Master CTA
- Test drugs by class, across many companies
- Shared cost of profiling
- Financial support separated from drug supply
- Shared IT Infrastructure, caBIG
- Protocol & ICF structure to minimize delays
|
| Use Team Approach | - Democratize access to data
- Share credit and opportunity
- Collaborative process for development
|
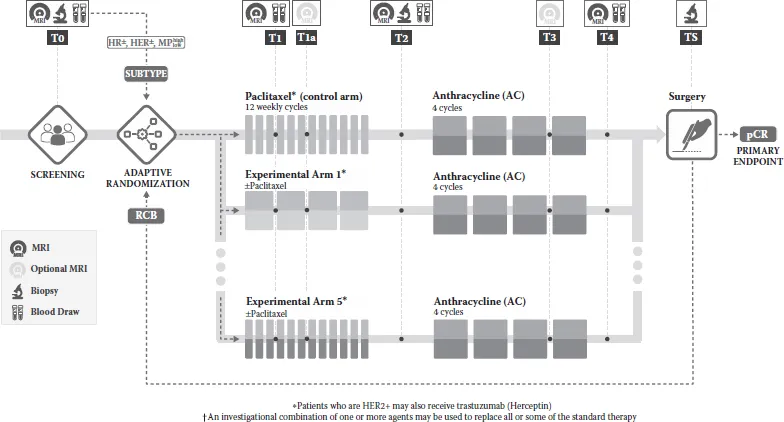
I-SPY2 is focused on the evaluation of new agents for neoadjuvant treatment of breast cancers with a high risk of recurrence. It is a precompetitive collaboration among multiple academic, pharmaceutical, bio-technology, governmental, and advocate stakeholders. New agents are administered in combination with standard neoadjuvant therapy (which serves as the common control arm) consisting of 12 weekly cycles of paclitaxel, followed by 4 cycles of anthracycline-based chemotherapy (Figure 1.1).
FIGURE 1.1
Study schema for t...