
eBook - ePub
Handbook of Semiconductor Electrodeposition
R.K. Pandey, S.N. Sahu, S. Chandra
This is a test
Share book
- 304 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Handbook of Semiconductor Electrodeposition
R.K. Pandey, S.N. Sahu, S. Chandra
Book details
Book preview
Table of contents
Citations
About This Book
Aiming to bridge the gap in understanding between professional electrochemists and hard-core semiconductor physicists and material scientists, this book examines the science and technology of semiconductor electrode-positioning. Summarizing state-of-the-art information concerning a wide variety of semiconductors, it reviews fundamental electrodeposition concepts and terminology.
Frequently asked questions
How do I cancel my subscription?
Can/how do I download books?
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
What is the difference between the pricing plans?
Both plans give you full access to the library and all of Perlego’s features. The only differences are the price and subscription period: With the annual plan you’ll save around 30% compared to 12 months on the monthly plan.
What is Perlego?
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Do you support text-to-speech?
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Is Handbook of Semiconductor Electrodeposition an online PDF/ePUB?
Yes, you can access Handbook of Semiconductor Electrodeposition by R.K. Pandey, S.N. Sahu, S. Chandra in PDF and/or ePUB format, as well as other popular books in Technologie et ingénierie & Ingénierie de l'électricité et des télécommunications. We have over one million books available in our catalogue for you to explore.
Information
1
Basic Concepts
Thin-film technology is the basis of astounding developments in solid-state electronics. The thin films can be obtained either by physical methods (e.g., thermal evaporation, sputtering, epitaxial growth) or by chemical methods (e.g., anodization, chemical vapor deposition (CVD), chemical bath, electrodeposition). Physical methods are expensive but give relatively more reliable and more reproducible results. Most of the chemical methods are cost-effective, but their full potential for obtaining device quality films has not been fully explored in many cases. Electroplating is the simplest of the chemical methods, and it is rather surprising that efforts to obtain good quality semiconductor films have only recently started.
The art of electroplating metals and metallic alloys has been in vogue for nearly a century, and the earlier efforts are well documented (Brenner 1963a,b; Bockris and Damjanovic 1964; Fleischman and Thirsk 1963; Lowenheim 1974; Duffy 1981; Blum and Hogaboom 1949). Most of the development has been more by way of art rather than science, which started to emerge only recently. Further, the viability of using the electrodeposition technique as a tool of materials technology is attracting attention as a means of obtaining films of a wide variety of materials including semiconductors, high-Tc superconductors, polymer films, materials for functional biostimulation (Ir2O3, Rb2O3, RuO2, etc.), specific electronic device application materials, and others.
Some of the key advantages of the electrodeposition technique are
- It is possible to grow uniform films over large areas, as well as irregularly shaped surfaces.
- Compositionally modulated structures or nonequilibrium alloys can be electroplated.
- A wide range of industrial experience can be drawn upon.
- It is specially attractive in terms of cost, high throughput, and scalability.
This monograph seeks to introduce the state of the art of electrodepositing elemental and compound semiconductor films, which is a development of the last two decades. Broadly speaking, the electrodeposition process encompasses physical as well as chemical phenomena that require an understanding of the following aspects:
- The influence of electric fields and ionic transport on the discharge process.
- Thermodynamic and kinetic factors related to the electrodeposition process.
- Factors controlling composition, structure, and morphology.
- The role of additives during electrodeposition. What happens to them?
- The use of in situ and ex situ probes for the characterization of electrodeposits.
- What control schemes can be envisaged that will result in deposit properties that satisfy the standards set by the photovoltaic, electronic, and other industries?
I Basics of Electrodeposition
Electrodeposition of metallic films (commonly known as electroplating) has long been known and used for preparing metallic mirrors and corrosion-resistant surfaces, among other things. In its simplest form, the electroplating or electrodeposition bath consists of an electrolyte containing metal ions (for example, CuS04 solution for the deposition of copper), an electrode or substrate on which the deposition is desired, and a counter electrode. When a current flows through the electrolyte, the cations and anions move toward the cathode and anode, respectively, and may deposit on the electrodes after undergoing a charge transfer reaction. Suppose copper is to be electrodeposited. Then the electrodeposition bath could contain some Cu salt such as copper sulfate as shown in Fig. 1.1. The Cu2+ ions deposit on the substrate, and a copper coating is obtained. Historically, the discovery of electroplating can be traced back to Michael Faraday and his famous laws of electrolysis. Faraday’s laws can be stated as follows.
Faraday’s First Law. The total amount of chemical change produced by an electric current is proportional to the total charge passing through the electrolyte.
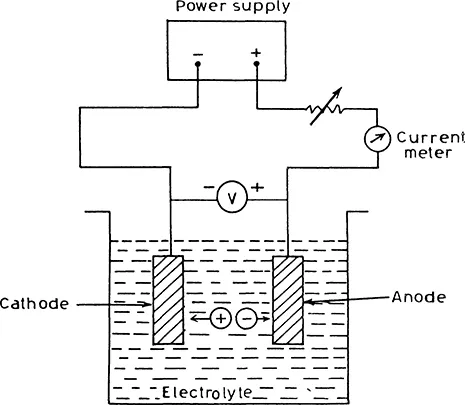
Figure 1.1 Schematic representation of a simple electrodeposition bath.
Faraday’s Second Law. The masses of the different substances liberated in the el...