
eBook - ePub
Handbook of Semiconductor Electrodeposition
R.K. Pandey, S.N. Sahu, S. Chandra
This is a test
Partager le livre
- 304 pages
- English
- ePUB (adapté aux mobiles)
- Disponible sur iOS et Android
eBook - ePub
Handbook of Semiconductor Electrodeposition
R.K. Pandey, S.N. Sahu, S. Chandra
Détails du livre
Aperçu du livre
Table des matières
Citations
À propos de ce livre
Aiming to bridge the gap in understanding between professional electrochemists and hard-core semiconductor physicists and material scientists, this book examines the science and technology of semiconductor electrode-positioning. Summarizing state-of-the-art information concerning a wide variety of semiconductors, it reviews fundamental electrodeposition concepts and terminology.
Foire aux questions
Comment puis-je résilier mon abonnement ?
Il vous suffit de vous rendre dans la section compte dans paramètres et de cliquer sur « Résilier l’abonnement ». C’est aussi simple que cela ! Une fois que vous aurez résilié votre abonnement, il restera actif pour le reste de la période pour laquelle vous avez payé. Découvrez-en plus ici.
Puis-je / comment puis-je télécharger des livres ?
Pour le moment, tous nos livres en format ePub adaptés aux mobiles peuvent être téléchargés via l’application. La plupart de nos PDF sont également disponibles en téléchargement et les autres seront téléchargeables très prochainement. Découvrez-en plus ici.
Quelle est la différence entre les formules tarifaires ?
Les deux abonnements vous donnent un accès complet à la bibliothèque et à toutes les fonctionnalités de Perlego. Les seules différences sont les tarifs ainsi que la période d’abonnement : avec l’abonnement annuel, vous économiserez environ 30 % par rapport à 12 mois d’abonnement mensuel.
Qu’est-ce que Perlego ?
Nous sommes un service d’abonnement à des ouvrages universitaires en ligne, où vous pouvez accéder à toute une bibliothèque pour un prix inférieur à celui d’un seul livre par mois. Avec plus d’un million de livres sur plus de 1 000 sujets, nous avons ce qu’il vous faut ! Découvrez-en plus ici.
Prenez-vous en charge la synthèse vocale ?
Recherchez le symbole Écouter sur votre prochain livre pour voir si vous pouvez l’écouter. L’outil Écouter lit le texte à haute voix pour vous, en surlignant le passage qui est en cours de lecture. Vous pouvez le mettre sur pause, l’accélérer ou le ralentir. Découvrez-en plus ici.
Est-ce que Handbook of Semiconductor Electrodeposition est un PDF/ePUB en ligne ?
Oui, vous pouvez accéder à Handbook of Semiconductor Electrodeposition par R.K. Pandey, S.N. Sahu, S. Chandra en format PDF et/ou ePUB ainsi qu’à d’autres livres populaires dans Technologie et ingénierie et Ingénierie de l'électricité et des télécommunications. Nous disposons de plus d’un million d’ouvrages à découvrir dans notre catalogue.
Informations
Édition
11
Basic Concepts
Thin-film technology is the basis of astounding developments in solid-state electronics. The thin films can be obtained either by physical methods (e.g., thermal evaporation, sputtering, epitaxial growth) or by chemical methods (e.g., anodization, chemical vapor deposition (CVD), chemical bath, electrodeposition). Physical methods are expensive but give relatively more reliable and more reproducible results. Most of the chemical methods are cost-effective, but their full potential for obtaining device quality films has not been fully explored in many cases. Electroplating is the simplest of the chemical methods, and it is rather surprising that efforts to obtain good quality semiconductor films have only recently started.
The art of electroplating metals and metallic alloys has been in vogue for nearly a century, and the earlier efforts are well documented (Brenner 1963a,b; Bockris and Damjanovic 1964; Fleischman and Thirsk 1963; Lowenheim 1974; Duffy 1981; Blum and Hogaboom 1949). Most of the development has been more by way of art rather than science, which started to emerge only recently. Further, the viability of using the electrodeposition technique as a tool of materials technology is attracting attention as a means of obtaining films of a wide variety of materials including semiconductors, high-Tc superconductors, polymer films, materials for functional biostimulation (Ir2O3, Rb2O3, RuO2, etc.), specific electronic device application materials, and others.
Some of the key advantages of the electrodeposition technique are
- It is possible to grow uniform films over large areas, as well as irregularly shaped surfaces.
- Compositionally modulated structures or nonequilibrium alloys can be electroplated.
- A wide range of industrial experience can be drawn upon.
- It is specially attractive in terms of cost, high throughput, and scalability.
This monograph seeks to introduce the state of the art of electrodepositing elemental and compound semiconductor films, which is a development of the last two decades. Broadly speaking, the electrodeposition process encompasses physical as well as chemical phenomena that require an understanding of the following aspects:
- The influence of electric fields and ionic transport on the discharge process.
- Thermodynamic and kinetic factors related to the electrodeposition process.
- Factors controlling composition, structure, and morphology.
- The role of additives during electrodeposition. What happens to them?
- The use of in situ and ex situ probes for the characterization of electrodeposits.
- What control schemes can be envisaged that will result in deposit properties that satisfy the standards set by the photovoltaic, electronic, and other industries?
I Basics of Electrodeposition
Electrodeposition of metallic films (commonly known as electroplating) has long been known and used for preparing metallic mirrors and corrosion-resistant surfaces, among other things. In its simplest form, the electroplating or electrodeposition bath consists of an electrolyte containing metal ions (for example, CuS04 solution for the deposition of copper), an electrode or substrate on which the deposition is desired, and a counter electrode. When a current flows through the electrolyte, the cations and anions move toward the cathode and anode, respectively, and may deposit on the electrodes after undergoing a charge transfer reaction. Suppose copper is to be electrodeposited. Then the electrodeposition bath could contain some Cu salt such as copper sulfate as shown in Fig. 1.1. The Cu2+ ions deposit on the substrate, and a copper coating is obtained. Historically, the discovery of electroplating can be traced back to Michael Faraday and his famous laws of electrolysis. Faraday’s laws can be stated as follows.
Faraday’s First Law. The total amount of chemical change produced by an electric current is proportional to the total charge passing through the electrolyte.
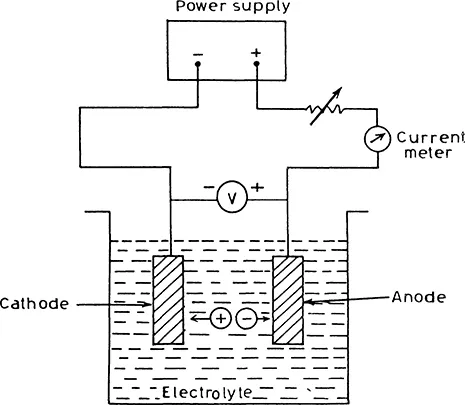
Figure 1.1 Schematic representation of a simple electrodeposition bath.
Faraday’s Second Law. The masses of the different substances liberated in the el...