CONTENTS
Norovirus
Introduction
History
Norovirus Is the Most Common Cause of Gastroenteritis
Rise of Norovirus
Norovirus Is the Dominant Cause of AGE in NSW, Australia
NoV Classification and Epidemiology
NoV Classification and Genetic Diversity
Molecular Epidemiology of NoV
Pandemic Noroviruses of the Genogroup II, Genotype 4 Lineage
Epidemic Noroviruses
Norovirus Structure and Genome Organization
NoV Nonstructural Proteins Involved in Genome Replication
NoV Structural Proteins
Replication and Life Cycle of NoV
NoV Binding and Cell Entry
Translation of Viral Proteins
Norovirus Evolution
Antigenic Variation NoV Evolution
RNA Recombination
Nomenclature of NoV Recombinants
Norovirus Clinical Disease
Pathogenesis
Immunity
Virus–Host Interaction and Innate Immunity
Host Susceptibility Factors
Transmission
Non-Foodborne Outbreak Settings
Norovirus Transmission from Food and Water
Transmission from Seafood
Norovirus Transmission from Food Handlers
Transmission from Contaminated Water
Transmission from Fresh Produce
NoV Prevention and Control
Vaccine Development
Norovirus: Conclusions
Sapovirus
Introduction
Classification and History
Genome Organization
Detection Methods
Pathogenesis and Clinical Manifestation
Environmental Persistence
Epidemiology
Transmission of Infection
Foodborne Transmission
Sapovirus: Conclusions
References
NOROVIRUS
Introduction
Human norovirus (NoV) is the leading cause of acute gastroenteritis (AGE) globally [1,2], and carries with it a large social and financial burden resulting from forced time off work, business closures, and hospitalizations [3]. Foodborne transmission is a key route for NoV infection, as the virus is spread rapidly via contaminated food handlers and food products. In many developed nations, NoV has been identified as the leading cause of foodborne disease, accounting for more than half of the cases of foodborne illness each year [4, 5, 6 and 7]. As the etiological agent responsible for the majority of foodborne illness (up to 80%) is never identified, estimates additionally attribute NoV with a large proportion of these unresolved cases [4].
NoV remains challenging to develop vaccinations against, with the virus continually mutating to evade herd immunity. Human NoV is also very difficult to culture in immortalized cells, severely hampering efforts to develop treatments and vaccines against the virus [8]. Without a reliable animal model to study human NoV directly, most studies rely on murine norovirus (MNV), a genogroup V NoV. MNV has been used to infer details of the infection cycle, including viral cell entry, and is frequently used to study aspects of NoV replication in both cell culture and in vivo mouse studies [9,10].
NoVs are classified within the Caliciviridae family and named after calyx (the Greek word for cup) because of the cuplike pattern on the virion surface [1]. Of the six Caliciviridae genera, NoV is attributed with the highest incidence of human disease [3]. Symptoms of NoV infection include vomiting and diarrhea, often accompanied by nausea, stomach cramps, malaise, and fever [11], which follow a relatively short incubation period of 24–48 h. Symptoms usually persist for 2–4 days in healthy adults [12], but infections can persist for months or even years in immunosuppressed or immunocompromised individuals [13, 14 and 15]. While the main route of NoV transmission is person-to-person [16], foodborne NoV is another significant route [3]. This section of the chapter reviews NoV and its transmission via contaminated food and water.
HISTORY
NoV was first described as hyperemesis hiemis or winter vomiting disease by Zahorsky in 1929 [17,18]. However, the disease was not fully understood at that time [19]. In the 1970s, several transmissibility studies in volunteer subjects who were orally administered stool filtrates obtained from infected patients showed that they developed similar gastroenteritis symptoms [20, 21 and 22]. These studies demonstrated that an unknown nonbacterial agent was the primary cause of transmissible AGE.
The original Norwalk virus was discovered in a fecal sample obtained from an AGE outbreak at an elementary school in Norwalk, Ohio, in 1968. The outbreak had a high attack rate of 50% (116 of 232) among the schoolchildren [23,24]. Norwalk virus, subsequently named after the town where it was found, is the prototype of the Norovirus genus. However, it took four more years before the actual physical virus particle was first visualized by Kapikian et al. in 1972 using electron microscopy (EM) [25].
Following the discovery of Norwalk virus, other similar viruses were subsequently detected and collectively termed Norwalk-like viruses (NLV) or small round structured viruses (SRSV) [26]. These viruses were typically named after the location where they were identified or by their appearance as visualized with EM [27,28]. Norwalk virus, the prototype NoV, is classified as NoV genogroup I, genotype 1 (GI.1). Complete sequencing and characterization of the Norwalk virus genome was accomplished by Jiang et al. in the early 1990s [29]. This achievement allowed further investigation into viral taxonomy using molecular techniques, including reverse transcription polymerase chain reaction (RT-PCR) and sequencing. In 2002, NLVs were collectively renamed as a single genus, Norovirus [30]. In summary, NoV-associated AGE with vomiting was recognized for many years before the actual virus was finally discovered. The true health burden of NoV has really only come to light in recent years.
NOROVIRUS IS THE MOST COMMON CAUSE OF GASTROENTERITIS
Rise of Norovirus
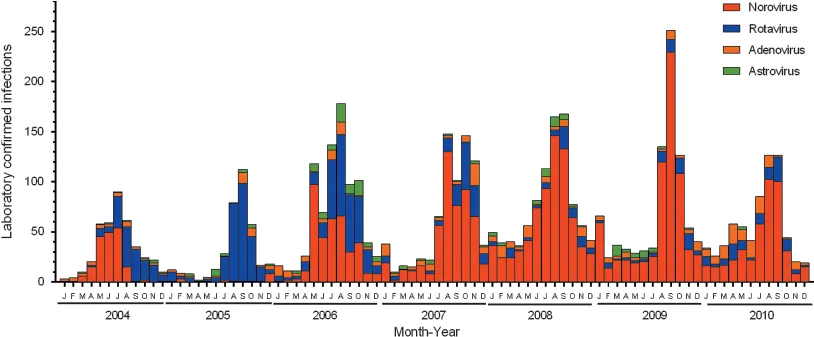
Historically, rotavirus (RoV) was reported as the major cause of diarrhea in children globally and was estimated to kill more than 440,000 children each year [31]. However, since 2006, the vaccines Rotarix (GlaxoSmithKline) and RotaTeq (Merck) have been introduced into the health-care programs of many countries. Following their inclusion, a dramatic decrease (~70%) in the incidence of RoV-associated AGE has been reported in these countries [32, 33, 34 and 35]. A similar trend has been seen in New South Wales (NSW), Australia, where the incidence of RoV infections decreased substantially following the introduction of the RoV vaccine in 2007, with the last major epidemic in 2005 (Figure 6.1).
Norovirus Is the Dominant Cause of AGE in NSW, Australia
In Australia, the first NoV foodborne-associated outbreak was reported in 1978 and was linked to oyster consumption [37]. The etiological agent, NoV, was confirmed by visualization of virus particles in 39% of fecal specimens by EM and in 75% of samples by immune-EM [37].
To determine the role of NoV as an etiological agent of AGE, a total...