
eBook - ePub
Bioresorbable Scaffolds
From Basic Concept to Clinical Applications
- 533 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Bioresorbable Scaffolds
From Basic Concept to Clinical Applications
About this book
This book focuses on the coronary bioresorbable scaffold, a new interventional treatment for coronary artery disease, differentiated from a permanent metallic stent. The book provides an overview of the technology including non-clinical studies and clinical evidences in order to help clinicians understand the appropriate application of the technology and the optimal techniques of implantation. It covers the basics of bioresorbable scaffolds; bench test results; preclinical studies; clinical evidences; and tips and tricks of implantation.
Information
1Introduction
1.1Early development of bioresorbable scaffold
Patrick W.J.C. Serruys as interviewed by Carlos Collet and Yoshinobu Onuma
1.1Early development of bioresorbable scaffold
Patrick W.J.C. Serruys as Interviewed by Carlos Collet and Yoshinobu Onuma
In September 1986, I had the privilege to implant the first endoluminal prosthesis (wall stent), as we called the stent at that time, in our first patient in Rotterdam just a few months after the pioneering cases of Jacques Puel in Toulouse and Ulrich Sigwart in Lausanne. In 1986, there was not yet an official Ethics Committee established at the Thoraxcenter, and it was only with the blessing of the chairman of cardiology and the permission of Paul Hugenholtz and with the support of the chief of surgery Egbert Bos that I was allowed to implant the first stent. Following the implantation, I performed an angioscopy, as it was available in Europe at that time. I was shocked by the vision of the shiny metal embedded in this delicate structure that is a human coronary artery. I felt guilty to have introduced in that very delicate biological structure such a rough device as a self-expanding stent. Honestly, I left the cath-lab with a tremendous feeling of guiltiness, having the impression that I had carried out something irreversible that would leave this human coronary artery caged forever. As ominously predicted by our chief surgeon... by the third case, I experienced my first stent thrombosis despite extensive platelet antiaggregation and anticoagulation with aspirin, heparin, warfarin, persantine and reomacrodex. On the same, day I started to reflect on the fact whether it could be possible to scaffold a vessel without using a permanent metallic implant to keep the vessel largely patent.
Scaffold was the word used by Ulrich Sigwart to describe the stenting process since at that time the word “stent” was not even mentioned in any dictionary of the English language. I told my colleagues that it would be possible to achieve the same kind of results with a device that would disappear with time. Immediately, my colleague Wim van der Giessen mentioned that a structure made of a polymer such as poly-lactide could do the trick, having heard that Richard Stack at Duke University was working on a biodegradable stent. I met Richard Stack at the American Heart Association 1988 and for more than one hour we shared our interest in developing a new field of bioresorbable stenting and later on it became evident that it would be a very complex and difficult endeavour, since nobody else including manufacturers and major device companies like Schneider, Boston Scientific, or Cordis showed interest in the development of biodegradable technologies. They had enough issues on their hands with the bare metallic stents.
Back in the Netherlands, Wim van der Giessen and myself kept working on the concept of developing a stent with a biostable polymer with the major Dutch corporation AkzoNobel. The results of this scaffold implantation in the coronaries of the pig model were very satisfactory and were published in the Journal of Interventional Cardiology in 1992 [1]. I will never forget the visit of the chief of cardiology Paul Hugenholtz, to the headquarters of AkzoNobel Company, who specialised in polymers to encapsulate the telephonic and electrical cable under the Atlantic Ocean for telecommunication between Europe and the United States. When I mentioned to the CEO of the company that a stent had on average a length of 14 mm and a diameter of 3 mm, the discussion came quickly to an end and clearly the microscopic need of polymers to manufacture a stent did not excite him excessively and did not steer his imagination or his vision as a captain of the industry.
In 1995, I went to the Mayo and Cleveland Clinics. We created a working group to test a family of bioresorbable polymers and biostable polymers, all attached on the external surface of a Wictor stent. I guess that the model was inappropriate, creating an eccentric and asymmetric configuration of the implant that induced major mechanical injury and inflammatory reactions. It was with anxiety that we all met at the American Heart Association in 1996, each academic group having experienced terrible animal results and we concluded that it was not the way to go. In the year 2000, Dr. Igaki and Dr. Tamai came to Rotterdam and in a meeting at the Hilton Hotel showed me a self-expanding thermolabile biodegradable scaffold, the so-called Igaki-Tamai stent. I will never forget the demonstration in the bathroom of their hotel, filing a glass with hot water from the tap and then dropping the stent into the glass and then expanding. Convinced by this experience and seeing for the first time a clinical product, we convinced the ethics committee to conduct a short series of 10 clinical implantations. As the matter of fact, we carried out the implantation with Dr. Tamai during his stay in Rotterdam, and unfortunately by the seventh case the device got somewhat dislodged from the balloon and subsequently was pulled out with the balloon semi-inflated, which was a good reason to stop working with this device. The reader has to understand that we had to inflate the balloon with hot contrast medium at 60°C to obtain thermolabile self-expansion which was another reason to terminate the clinical trial. Dr. Tamai had the courage to complete a series of 55 patients and 10 years later we published together, after his death, the results of the 10 years’ follow-up of these patients in Circulation Journal [2].
July 1999 was for me the discovery of stents with a permanent coating eluting called Rapamycin at the headquarters of Cordis Johnson and Johnson. Our first experience was in Rotterdam and in Sao Paulo with the Cypher® Stent. And of course, the Cypher stent for a few years overshadowed any development in the field of biodegradable scaffolds. It is Hans Bonier, one of my ex-trainees, who drew my attention to the idea of a German Professor of Cardiology, Heublein, who had developed the concept of a metallic bioresorbable stent using magnesium. I found the idea fascinating, but obviously, Biotronic being a German company, the technology went to Raymund Erbel and Michael Haude in order to be investigated. I followed the development and gave my advice to them on several occasions. So when Abbott made known that they were ready with their first bioresorbable scaffold, the so-called ABSORB Cohort A, the first iteration of an everolimus bioresorbable scaffold, I was fully committed to investigate that product the best I could. John Ormiston had the privilege to perform the first historical implantation. At the Thoraxcenter, we implanted 16 of the first 30 scaffolds. And from the very beginning, I used the combination of optical coherence tomography, virtual histology, grayscale intravascular ultrasound, angiography, and multislice CT scan to assess the results (Figure 1.1.1). Later on, it appeared that this combination of invasive and noninvasive imaging was essential and critical in the understanding of the bioresorbable concept. Yoshi Onuma joined me at that time and became instrumental in collecting and analyzing the 6-month and 2-year results of the Cohort A. We came to the conclusion that the integrity of the device was subsiding too quickly and could not counteract fully the constrictive reaction of the vessel wall following the barotrauma of the implantation. A second iteration was designed since the late loss with the first device was 0.44 mm, a loss judged by myself unacceptable, taking into account that even a stent like the Taxus® stent had a loss of 0.39 mm [3,4]. The second generation, the so-called ABSORB Cohort B, was a real success in the sense that we lowered the late loss to 0.23 mm with long-term results that were mimicking the results of Cohort A [5]. From Cohort A, although the results at 6 months were not acceptable, we learned that the later follow-up with positive remodeling and late lumen enlargement had tremendous potential and should be duplicated by this second iteration device, providing a better late loss could be obtained. This was going to be achieved with Cohort B1 and B2.
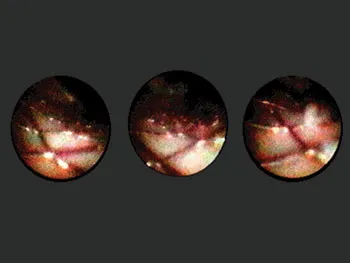
Figure 1.1.1Angioscopy of a coronary artery with a wall stent implanted showing prolapse of tissue trough the struts.
As usual, I have been extremely optimistic about new technology and made some overenthusiastic stateme...
Table of contents
- Cover
- Half Title Page
- Title Page
- Copyright Page
- Contents
- Author
- Part 1 Introduction
- Part 2 Principles of bioresorption, vascular application
- Part 3 From bench test to preclinical assessment
- Part 4 Lessons learned from preclinical assessment
- Part 5 Imaging to evaluate the bioresorbable scaffold: A core lab perspective: Methodology of measurement and assessment
- Part 6 Clinical evidence of randomized and nonrandomized trials: Personal perspective
- Part 7 Clinical evidence in specific patient subsets: Personal perspective
- Part 8 Complications (incidence, diagnosis, potential mechanisms and treatment)
- Part 9 Tips and tricks to implant BRSs
- Part 10 Emerging technologies (pre-CE mark, pre-FA, pre-PMDA, and pre-CFDA)
- INDEX
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Bioresorbable Scaffolds by Yoshinobu Onuma, Patrick W.J.C. Serruys, Yoshinobu Onuma,Patrick W.J.C. Serruys in PDF and/or ePUB format, as well as other popular books in Medicine & Biotechnology in Medicine. We have over one million books available in our catalogue for you to explore.