
eBook - ePub
NMR and Chemistry
An introduction to modern NMR spectroscopy, Fourth Edition
- 400 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
Keeping mathematics to a minimum, this book introduces nuclear properties, nuclear screening, chemical shift, spin-spin coupling, and relaxation. It is one of the few books that provides the student with the physical background to NMR spectroscopy from the point of view of the whole of the periodic table rather than concentrating on the narrow applications of 1H and 13C NMR spectroscopy. Aids to structure determination, such as decoupling, the nuclear Overhauser effect, INEPT, DEPT, and special editing, and two dimensional NMR spectroscopy are discussed in detail with examples, including the complete assignment of the 1H and 13C NMR spectra of D-amygdain.
The authors examine the requirements of a modern spectrometer and the effects of pulses and discuss the effects of dynamic processes as a function of temperature or pressure on NMR spectra. The book concludes with chapters on some of the applications of NMR spectroscopy to medical and non-medical imaging techniques and solid state chemistry of both I = F1/2 and I > F1/2 nuclei. Examples and problems, mainly from the recent inorganic/organometallic chemistry literature support the text throughout. Brief answers to all the problems are provided in the text with full answers at the end of the book.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access NMR and Chemistry by J.W. Akitt,B. E. Mann in PDF and/or ePUB format, as well as other popular books in Naturwissenschaften & Analytische Chemie. We have over one million books available in our catalogue for you to explore.
Information
The theory of nuclear magnetization 1
1.1 The Properties of the Nucleus of an Atom
The chemist normally thinks of the atomic nucleus as possessing only mass and charge and is concerned more with the interactions of the electrons that surround the nucleus, neutralize its charge and give rise to the chemical properties of the atom. Nuclei, however, possess several other properties that are of importance to chemistry, and to understand how we use them it is necessary to know something more about them.
Nuclei of certain natural isotopes of the majority of the elements possess intrinsic angular momentum or spin, of total magnitude . The largest measurable component of this angular moment is Iℏ, where I is the nuclear spin quantum number and ℏ is the reduced Planck’s constant, h/2π. The spin quantum number I may have integral or half-integral values (0, 1/2, 1, 3/2, . . .), the actual value depending upon the isotope. Since I is quantized, several discrete values of angular momentum may be observable and their magnitudes are given by ℏm where the quantum number m can take the values I, I – 1, 1 – 2, ..., –I. There are thus 2I + 1 equally spaced spin states of a nucleus with angular momentum quantum number I.
A nucleus with spin also has an associated magnetic moment μ. We define the components of μ associated with the different spin states as mpμI, so that μ, also has 2I + 1 components. In the absence of an external magnetic field the spin states all possess the same potential energy, but they take different energy values if a magnetic field is applied. The origin of the nuclear magnetic resonance (NMR) technique lies in these energy differences, though we must defer further discussion of this until we have defined some other basic nuclear properties.
The magnetic moment and angular momentum behave as if they were parallel or antiparallel vectors, i.e. pointing in the same or opposite directions. It is convenient to define a ratio between them which is called the magnetogyric ratio, γ:
γ has a characteristic value for each magnetically active nucleus and is positive for parallel and negative for antiparallel vectors. We will see that the sign of γ influences both spin–spin coupling and the way energy is exchanged between spins.
If I> 1/2 the nucleus possesses in addition an electric quadrupole moment, Q. This means that the distribution of charge in the nucleus is non-spherical and that it can interact with electric field gradients arising from the electric charge distribution in the molecule. This interaction provides a means by which the nucleus can exchange energy with the molecule in which it is situated and affects certain NMR spectra profoundly.
Some nuclei have I = 0. Important examples are the major isotopes 12C and 16O, which are both magnetically inactive – a fact that leads to considerable simplification of the NMR spectra of organic molecules. Such nuclei are, of course, free to rotate in the classical sense, but this must not be confused with the concept of quantum-mechanical ‘spin’. The nucleons, i.e. the particles such as neutrons and protons which make up the nucleus, possess intrinsic spin in the same way as do electrons in atoms. Nucleons of opposite spin can pair, just as do electrons, though they can only pair with nucleons of the same kind. Thus in a nucleus with even numbers of both protons and neutrons all the spins are paired and I = 0. If there are odd numbers of either or of both, then the spin is non-zero, though its actual value depends upon orbital-type internucleon interactions. Thus we build up a picture of the nucleus in which the different resolved angular momenta in a magnetic field imply different nucleon arrangements within the nucleus, the number of spin states depending upon the number of possible arrangements. If we add to this picture the concept that 5 bonding electrons have finite charge density within the nucleus and become partly nucleon in character, then we can see that these spin states might be perturbed by the hybridization of the bonding electrons and that information derived from the nuclear states might lead indirectly to information about the electronic system and its chemistry.
The most important properties of the elements relevant to NMR spectroscopy are listed in Table 1.1. This gives the atomic weight of the nuclear isotope of the element listed, and where there is more than one magnetically active isotope this is indicated by an atomic weight in parentheses. The isotope listed is the one most usually used, though the unlisted ones are in some cases equally usable. The next column gives the spin quantum number, I, followed by the natural abundance of the isotope. The receptivity or natural signal strength of the nucleus is given relative to that of 13C, which itself gives a fairly weak signal, and this figure is made up of the intrinsic sensitivity of the nucleus (high if the magnetic moment is high) weighted by its natural abundance. Some elements are used in enriched forms (particularly 2H and 17O), when the receptivity is, of course, substantially higher. The data are completed by the quadrupole moment (where I > 1/2) and the resonant frequency in a particular magnetic field. This can be determined to a much higher precision than shown, for a resonance in an individual compound, and it should be remembered that each nucleus will have a range of frequencies due to the chemical shift effect. The resonance frequency is proportional to the magnetogyric ratio.
Table 1.1 Nuclear properties of some of the elements
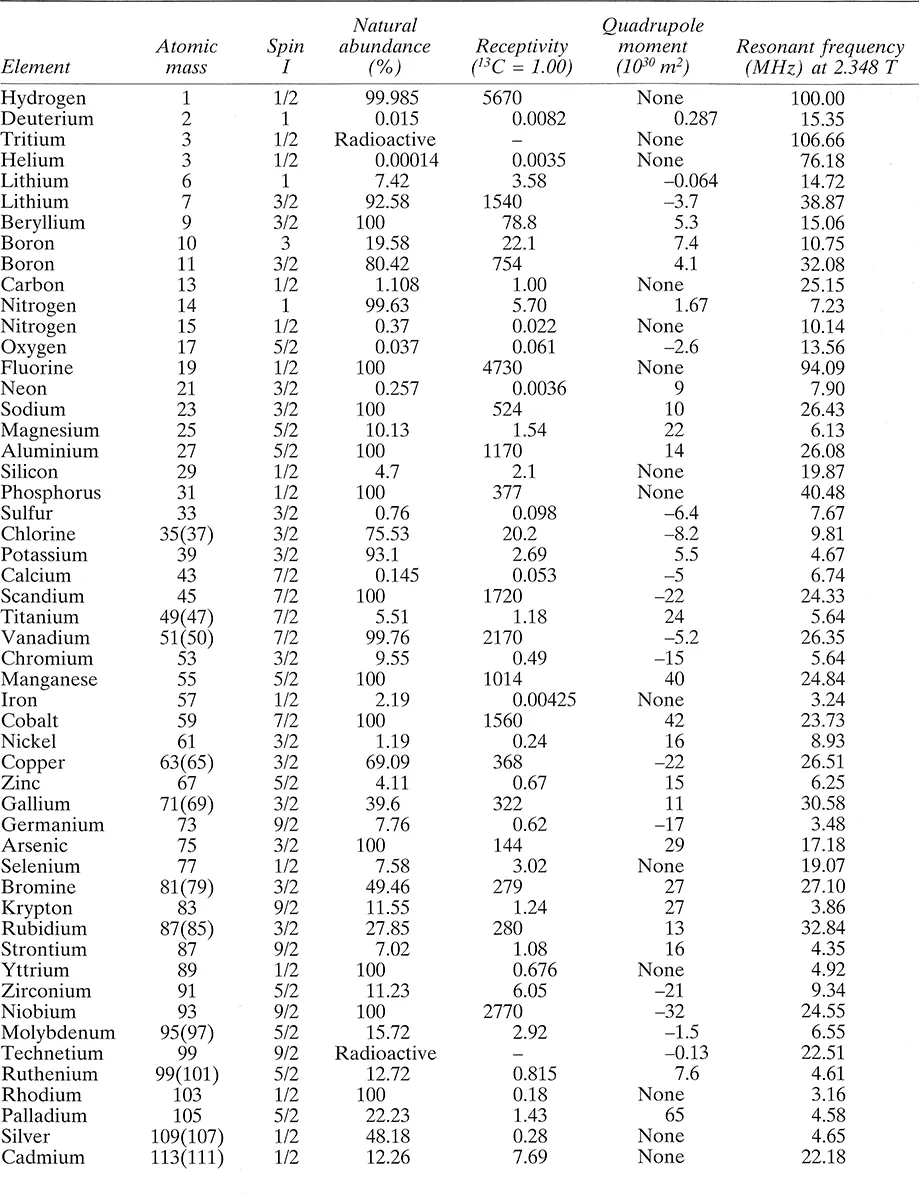
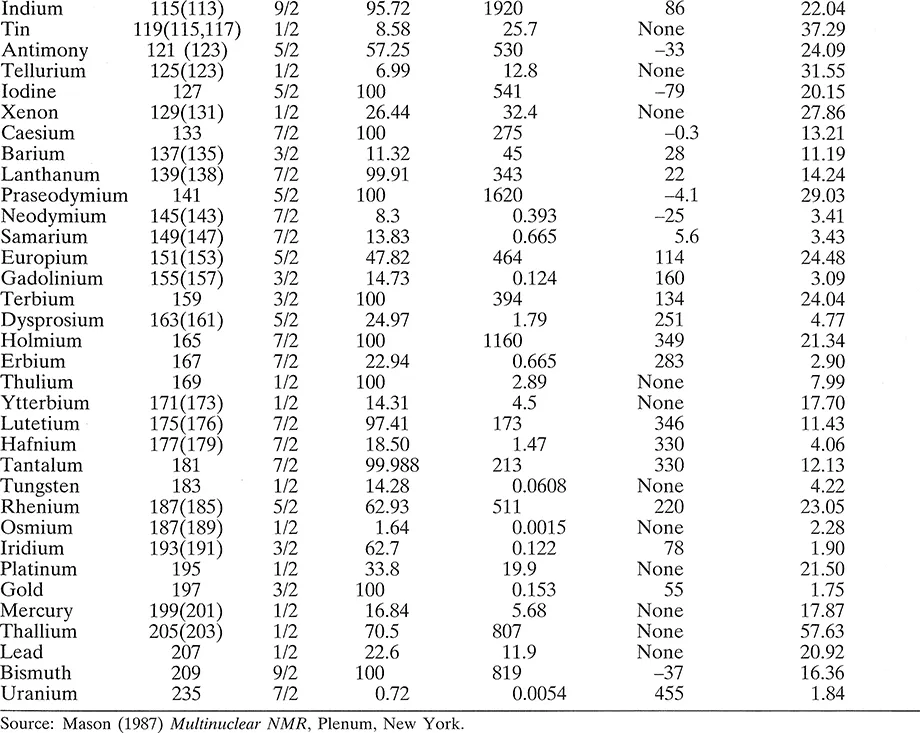
The first point to note about this list is that almost all the elements are represented, the only missing stable ones being argon and cerium, and that, in principle, virtually the whole of the Periodic Table can be studied by NMR. Indeed, with modern instrumentation, this is now realizable, though there are some cases, notably nuclei with very high quadrupole moments, where study in the liquid state is not rewarding. The usefulness of a nucleus to the NMR spectroscopist depends in the first place upon the chemical importance of the atom it characterizes and then upon its receptivity. Thus the extreme importance of carbon spectroscopy for understanding the structures of organic molecules has led to technical developments that have overcome the disadvantages of its poor receptivity, so that 13C NMR is now commonplace. Hydrogen, with its very high receptivity, has, of course, been studied right from the emergence of NMR spectroscopy as a technique useful to chemists, and proton spectroscopy, as it is often called (proton = 1H, the term is commonly used by NMR spectroscopists when discussing the nucleus of neutral hydrogen), has been used for the identification of the majority of organic compounds and many inorganic ones, and for the physical study of diverse sy...
Table of contents
- Cover
- Half Title
- Title Page
- Copyright Page
- Table of Contents
- Abbreviations
- Preface to the fourth edition
- 1 The theory of nuclear magnetization
- 2 The magnetic field at the nucleus: nuclear screening and the chemical shift
- 3 Internuclear spin–spin coupling
- 4 Nuclear magnetic relaxation
- 5 The spectrometer
- 6 Making the spins dance
- 7 NMR spectra of exchanging and reacting systems
- 8 Multiple resonance and one-dimensional pulse sequences
- 9 Two-dimensional NMR spectroscopy
- 10 Magnetic resonance imaging and biomedical NMR
- 11 High-resolution solid-state NMR
- Bibliography
- Answers to Questions
- Index