
- 624 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Introduction to Modern Inorganic Chemistry, 6th edition
About this book
This popular and comprehensive textbook provides all the basic information on inorganic chemistry that undergraduates need to know. For this sixth edition, the contents have undergone a complete revision to reflect progress in areas of research, new and modified techniques and their applications, and use of software packages.
Introduction to Modern Inorganic Chemistry begins by explaining the electronic structure and properties of atoms, then describes the principles of bonding in diatomic and polyatomic covalent molecules, the solid state, and solution chemistry. Further on in the book, the general properties of the periodic table are studied along with specific elements and groups such as hydrogen, the 's' elements, the lanthanides, the actinides, the transition metals, and the "p" block. Simple and advanced examples are mixed throughout to increase the depth of students' understanding.
This edition has a completely new layout including revised artwork, case study boxes, technical notes, and examples. All of the problems have been revised and extended and include notes to assist with approaches and solutions. It is an excellent tool to help students see how inorganic chemistry applies to medicine, the environment, and biological topics.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Introduction to Modern Inorganic Chemistry, 6th edition by R.A. Mackay,W. Henderson in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Chemistry. We have over one million books available in our catalogue for you to explore.
Information
CHAPTER 1 Introduction
1.1 INORGANIC CHEMISTRY AND THE DISCOVERY OF THE ELEMENTS
1.2 DEVELOPMENT
1.3 RECENT ADVANCES
1.4 INORGANIC NOMENCLATURE
1.5 APPROACH TO INORGANIC CHEMISTRY AND FURTHER READING
PROBLEMS
1.1 Inorganic chemistry and the discovery of the elements
Chemistry is one of the oldest and most wide-ranging of the sciences, and hence of human knowledge and endeavour. It had already grown sufficiently by the end of the 19th century to be conveniently divided into the three classical branches of inorganic, organic and physical chemistry. Inorganic chemistry covers the properties and reactions of all the chemical elements apart from carbon—now exceeding 110.
A major theme of inorganic chemistry over the last two millennia has been the discovery and characterization of the elements themselves. This continues to the present day in the synthesis of ultra-high atomic number elements by high energy bombardment (Section 16.12).
The discovery of the elements is summarized in Table 1.1. If the pattern of discovery is plotted against time, a curve is obtained which mirrors the pattern of development in many sciences. A long slow period of completely empirical advance in the ancient world was followed by a phase mainly of preservation and rediscovery through the Arab alchemists and in India and China. For the century up to AD 1750, some of the basic ideas of what we now call chemistry were developed from more deliberate investigations. From AD 1750 up to the first half of the 20th century, there was a sharply accelerating pattern of discovery as theory and technique advanced in parallel. Within this period we see individual spurts reflecting specific advances, like the 18th-century studies of gases, the early 19th-century use of electrolysis to isolate the very active metals, or the recognition of the Rare Gas Group which gave five new elements in five years. Eventually the pace slowed, in the decade to 1940, because there were ‘no new worlds to conquer’ and all the elements up to uranium had been identified. This was not the end of the story, as it turned out that further posturanium elements could be synthesized. This phase is now slowing down, reflecting the decreasing intrinsic stability of the nuclei. Whether this is finally the end of the story of the elements is not yet clear (compare Section 16.12). The overall pattern, found in many other developing fields, is of empirical discovery, acceleration fuelled by the interaction of greater understanding and improved methods, then maturity when the pace of change slows. Often, new accelerations start up from the mature phase, as unexpected observations or new ideas trigger off further developments.
1.2 Development
While we have followed the tale of the elements, the growth of inorganic chemistry as a whole followed a similar pattern. Inorganic chemistry was the first of the chemical sciences to flower in the course of the Scientific Revolution, and most of the work leading to the formulation of the atomic theory was carried out on inorganic systems, especially the gases and simple compounds like the nitrates, carbonates or sulfates. A critical advance in technique was the development of ever more accurate measures of the quantity of material—both by weighing and by measurement of gases. Once it could be established that a particular substance had the same composition when prepared by different routes (for example, an oxide prepared from the metal and air, from heating the carbonate, from precipitating the hydroxide from solution and igniting) the way was open to following changes quantitatively, to formulating generalizations like ‘the Law of Constant Composition’, and ultimately to Dalton’s atomic theory. It is worth remarking that even the most sophisticated modern experiment depends ultimately on accurate measurement of weight changes.
TABLE 1.1 The discovery of the elements
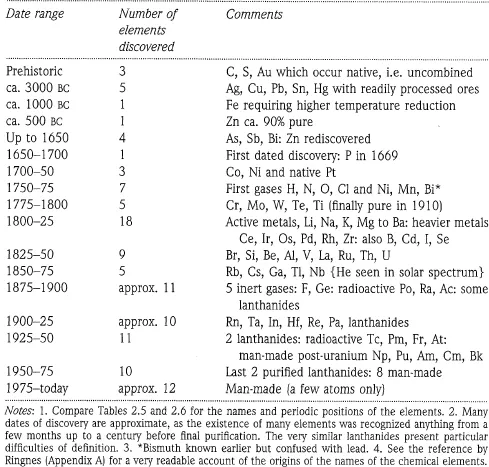
In the first half of the 19th century, not only had more than half of the elements been isolated but a great many of their simpler compounds had been studied. It is remarkable that explosive nitrogen trichloride or highly corrosive hydrogen fluoride were under study around 1800. By contrast, only a few simple organic compounds were known by 1820 and little progress was being made in organic chemistry as much of the effort was directed to extremely complex materials like milk or blood. By the middle of the 19th century came the period of spectacular advance in organic chemistry, followed around 1900 by a great upsurge of interest in physical chemistry. These advances meant nearly a century of comparative neglect of inorganic chemistry.
BRANCHES OF CHEMISTRY
Since the chemistry of one element, carbon, is so enormously ramified—probably similar in extent to the total chemistry of the remaining 109 put together—it has traditionally made up the separate field of organic chemistry. The bridging discipline covering the organic chemistiy of the inorganic elements, organoelement or organometallic chemistry, is an extensive field to which we shall make substantial reference. In addition, part of the chemistiy of carbon, covering the element and simpler compounds like its oxides and oxyions or the carbides, is traditionally covered in inorganic chemistry. No attempt is made to draw rigid boundaries.
The detailed study of energy changes, reaction mechanisms, much of bonding theory, the chemistiy of polymers, chemistry which occurs at surfaces and interfaces, the behaviour of metallic systems—all these fall into physical chemistry. Again, there are no rigid demarcations, and much of the most exciting work is done at the points of overlap. Other longstanding subdivisions include analytical chemistry and theoretical chemistry.
As might be expected, the huge expansion of chemistry in the last few decades has led to further subdivision within inorganic chemistry—such as phosphorus chemistry or transition metal chemistiy—as well as the defining of new fields with substantial overlap with inorganic chemistry. The latter include materials science, catalysis, inorganic biochemistry, computational chemistry and many more.
The Roots of Inorganic Chemistry
The origins of inorganic chemistry are ancient. Observation followed by what we now describe as empirical experiments led to the slow development of new materials from the early stages in human history. Thus beads of glass and of ceramics are found in ancient Egyptian burials and pottery was made by the earliest civilizations. Considerable control was achieved: black or red pottery was made by reducing or increasing the proportion of air, and colours and glazes had developed to a high degree of sophistication by 500 BC.
The discovery of metal extraction and processing was a most important theme and led to substantial mastery of the technologies by ancient craftsmen. Gold is usually found naturally as the free metal and, as shown by grave goods, has always been highly valued. Modern analysis of ancient artefacts shows that it was understood that the addition of small proportions of silver or copper gave a harder, more wear-resisting metal, and gave desirable variations in colour. The metal contents of gold coins held to highly consistent standards which correlated through many countries in a chain of related weights and gold contents—for example, from Macedonia to India in the 5th century BC. Copper has been known for about 7000 years and has been extracted from sulfide ores for around 5500 years. Small metal items like beads and bracelets are found in graves for several centuries before larger products like tools or weapons, suggesting a period where manufacture was difficult and not well understood. Analysis on ancient kilns shows that temperatures up to 1200°C were achieved in Bronze age copper smelting. Once production methods had evolved to the larger scale, it was rapidly found that alloying copper with tin to give bronze, or with zinc to give brass, produced metals of superior properties.
Tin, which is fairly inert to air, was probably prepared in a pure form many centuries before the more reactive zinc. Iron requires much more sophisticated treatment, and can be extracted only at the high temperatures achieved with air blown through a bed of charcoal. The carbon is also necessary to remove the oxygen from the ore. Even so, early temperatures did not reach the melting point, so casting was not possible and the metal was shaped by hammering. As iron weapons are much superior to bronze, it is thought that the initial discovery was kept secret for a millennium and knowledge of iron only became widespread after the destruction of the Hittite empire about 1200 BC (recent archaeology suggests this picture is oversimplified). Compared with iron, isolation of the other elements found up to the 18th century is relatively easy, so iron-working represents a peak in technological achievement which lasted for something like 4000 years.
Of course, very important advances were made, including the formulation of the Periodic Table, the discovery and exploitation of radiochemistry, and the classical work on non-aqueous solvents and on the complex chemistry of the transition elements, but it was not until the 1930s that the modern upsurge of interest in inorganic chemistry got under way. Among the seeds of this renaissance were the work of Stock and his school on volatile hydrides of boron and silicon, of Werner and others on the chemistry of transition metal complexes, of Kraus and Walden on non-aqueous solvents, and the work of a number of groups on radioactive decay processes. At the same time, the theories which play an important part in modern inorganic chemistry were being formulated and applied to chemical problems. The discovery of the fundamental particles and the structure of the atom culminated in the development of wave mechanics, which is the basis of all modern approaches to valency and bonding. This theory is outlined in Chapter 2, and its application to molecular structure is given in Chapters 3 and 4. A little later, the effect of an atom’s environment on the energy of its d electrons was brought into the treatment of transition metal compounds in the crystal field theory which is discussed in Chapter 13.
1.3 Recent advances
All these developments prepared the ground for the expansion of inorganic chemistry, starting in the 1950s, which was stimulated both by developments on the academic side in experiment and theory, and by the demand for new materials and for knowledge of many elements hitherto scarcely studied.
The advent of atomic energy focused attention on heavy transition elements and lanthanides (for example, the chemically very similar Zr and Hf have quite different neutron absorption properties). Similarly, the growth of electronics, followed by computers, led to growth in the chemistry of lesser-known Main Group elements involved in semiconductors, such as Ge, Ga, In and Se. A further significant change in the latter half of the 20th century was the very rapid growth in the number of working scientists and technologists, allowing simultaneous growth throughout chemistry. In earlier times, fields expanded only at the price of relative neglect in other areas.
Starting in the 1950s, transition element chemistry grew from 10% of Honours courses to become the dominant area of interest for several decades, largely as a result of the strong mutual stimuli of experimental and theoretical advances. More recent growth has encompassed fields such as low oxidation state chemistry, organometallic compounds, metal cluster chemistry, dendrites and other macromolecules, and multiple metal-metal bonding (compare Chapter 16). A decade or so later came a similar expansion in Main Group chemistry, building from the more traditional compounds into a substantial range of new species, including rare gas compounds, compounds containing chains, rings or clusters of like atoms, and unusual oxidation states stabilized by specially designed ligands (compare Section 17.9 and Chapter 18). All these developments were led by advances in preparative chemistry and in efficient methods for separating and rapidly characterizing new compounds (see Chapter 7).
Far from slackening, the pace has further increased. Through the turn of the millenium, we live with a continuing headlong expansion in inorganic chemistry, fuelled by new methods, new theories, new fields of interest like metals in biological systems, the search for new materials, new catalysts, more output for less pollution, and many other driving forces. Even unstable elements like technetium are finding uses in medicine, and highly radioactive isotopes, such as americium-241, find a variety of applications, including hous...
Table of contents
- Cover Page
- Half title
- Title Page
- Copyright Page
- Contents
- Preface
- SI Units and Names
- Chapter 1 Introduction
- Chapter 2 The Electronic Structure and the Properties of Atoms
- Chapter 3 Covalent Molecules: Diatomics
- Chapter 4 Polyatomic Covalent Molecules
- Chapter 5 The Solid State
- Chapter 6 Solution Chemistry
- Chapter 7 Experimental Methods
- Chapter 8 General Properties of the Elements in Relation to the Periodic Table
- Chapter 9 Hydrogen
- Chapter 10 The ‘s’ Elements
- Chapter 11 The Scandium Group and the Lanthanides
- Chapter 12 The Actinide Elements
- Chapter 13 The Transition Metals: General Properties and Complexes
- Chapter 14 The Transition Elements of the First Series
- Chapter 15 The Elements of the Second and Third Transition Series
- Chapter 16 Transition Metals: Selected Topics
- Chapter 17 The Elements of the ‘p’ Block
- Chapter 18 Selected Topics in Main Group Chemistry and Bonding
- Chapter 19 General Topics
- Chapter 20 Biological, Medicinal and Environmental Inorganic Chemistry
- Appendix A Further Reading
- Appendix B Some Common Polydentate Ligands
- Appendix C Molecular Symmetry and Point Groups
- Index
- Relative Atomic Masses
- Periodic Table of the Elements