
- 772 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Practical Fluorescence Spectroscopy
About this book
Presenting a detailed, hands-on approach to fluorescence spectroscopy, this book describes experiments that cover basic spectroscopy and advanced aspects of fluorescence spectroscopy. It emphasizes practical guidance, providing background on fundamental concepts as well as guidance on how to handle artifacts, avoid common errors, and interpret data. Nearly 150 experiments from biophysics, biochemistry, and the biomedical sciences demonstrate how methods are applied in practical applications. The result is a hands-on guide to the most important aspects of fluorescence spectroscopy, from steady-state fluorescence to advanced time-resolved fluorescence.
- Provides a complete overview of nearly 150 experiments using fluorescence spectroscopy, from basic to advanced applications
- Presents laboratory methods using a variety of instrumental setups with detailed discussion of data analysis and interpretations
- Covers steady-state phenomena, time-resolved phenomena, and advanced methods
- Spans biophysical, biochemical, and biomedical applications
- Describes related concepts, theory, and mathematical background as well as commercially available instruments used for measurements
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
CHAPTER 1
Theory of Light and Light Interaction with Matter
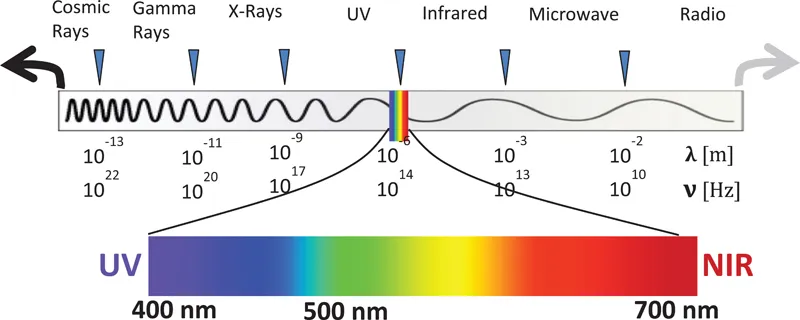
CONVENTIONALLY, WE CALL “LIGHT” a small range of a broad spectrum of electromagnetic radiation that corresponds to the visible spectral range (400–700 nm) as shown in Figure 1.1. Light is electromagnetic radiation which presents properties of both a wave and a particle. A “particle” of light is called a photon. The dual nature of light is often a source of confusion, but experiments confirming both interpretations exist. For example, experiments involving single-photon double-slit interference and the photoelectric effect have shown both the wave and particle nature of light, respectively. A photon is a quanta or the smallest possible amount/part of an electromagnetic energy/wave. Since we almost always refer to multiple photons, a photon can be referred to as a quantum of electromagnetic radiation. Both definitions will be used throughout this text. For example, a single chromophore (molecule) absorbs or emits a photon of a certain energy. It is important to remember that when discussing photons or electromagnetic waves, we are talking about light; both representations are equivalent, but one is often much easier applied to a particular scenario. For example, it is more natural to discuss a metal interacting with an oscillating electric wave rather than a stream of “particles” (photons) that never physically collide with the metal surface.
1-1 BASICS OF LIGHT
This section is meant as a review of the basics of light as electromagnetic waves or photons. More importantly, it should demonstrate that there are many different ways of looking at light from a mathematical perspective, but they all lead to the same interpretation. Various uses of light are presented across many different fields, especially biology, chemistry, physics, and engineering. Within each field, and indeed specializations within each individual field, the nomenclature and properties of interest vary. For example, in biology, spectra are frequently given in a wavelength scale (nanometers) but in the semiconductor field, they are typically presented on a scale in terms of energy (electron volts). However, any spectrum may be presented in wavelength or energy without a loss of information. By the end of this chapter, the reader should have a fluid understanding of the relationships between different notations.
When looking at the light, one most readily thinks about its brightness and color. The brightness of the light is determined by the intensity (energy), passing through a certain surface area orthogonal to the direction of light propagation per unit of time (we also call it photon flux). The intensity of light is typically denoted as I. A simple way of quantifying the brightness of the light is to think about how many photons hit a detector (e.g., human retina, charge-coupled device, photodiode) per unit time. The more photons that hit a detector, the brighter (i.e., more intense) the light source is. From the wave point of view, the intensity of the electromagnetic radiation is proportional to the square of the electric field amplitude. Color is related to the period (duration of time of one cycle of a wave) of the electromagnetic radiation; equivalently, color (wavelength) is related to the frequency or energy of the light wave.
Figure 1-1.1 shows a typical schematic of a light wave. The electromagnetic field is depicted as orthogonal electric and magnetic fields E and B, respectively. It should be noted that E and B are rarely drawn to scale as the amplitude of B is roughly 1/c the size of that of E. In Figure 1-1.1, the wave is traveling in the -direction, the electric field points in the -direction, and the magnetic field points in the -direction. It is important to notice that the electric field, magnetic field, and the direction of propagation are all orthogonal (akin to the “right-hand rule”). The electric field of light is given as:
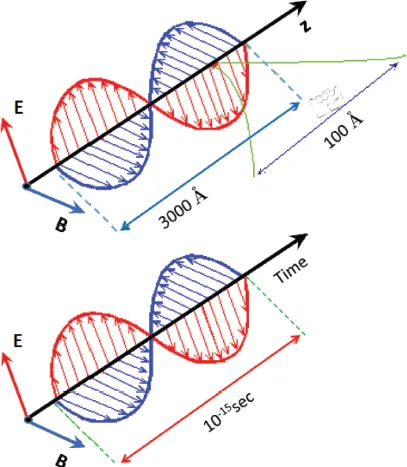
(1-1.1) |
E0 is the maximum intensity of the electric field or the amplitude of the electric vector and is the directional unit vector. This directional unit vector indicated allows for the direction of the electric field to point in any arbitrary direction. In the example in Figure 1-1.1, ; one may also see E0, a constant vector, in place of . In this case, , and . Thus, Equation 1-1.1 becomes:
(1-1.2) |
Thus, the intensity of this light ray is:
(1-1.3) |
where c is the speed of light, n is the refractive index of the medium the light is traveling in, and is the permittivity of vacuum. Light...
Table of contents
- Cover
- Half Title
- Title Page
- Copyright Page
- Table of Contents
- Preface
- Authors
- CHAPTER 1 ■ Theory of Light and Light Interaction with Matter
- CHAPTER 2 ■ Experimental Basics
- CHAPTER 3 ■ Steady State Experiments—Transmission/Absorption
- CHAPTER 4 ■ Fluorescence—Steady-State Phenomena
- CHAPTER 5 ■ Steady-State Fluorescence: Applications
- CHAPTER 6 ■ Steady-State Fluorescence Polarization: Anisotropy
- CHAPTER 7 ■ Fluorescence: Time-Resolved Phenomena
- CHAPTER 8 ■ Advanced Experiments
- INDEX
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Practical Fluorescence Spectroscopy by Zygmunt (Karol) Gryczynski,Ignacy Gryczynski in PDF and/or ePUB format, as well as other popular books in Medicina & Bioquímica en medicina. We have over one million books available in our catalogue for you to explore.