
- 214 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
This fourth edition of How Drugs Work equips readers with a set of clear concepts for matching the pharmacology to the diagnosis, and has been completely revised and updated to reflect the latest knowledge and terminology. Rather than providing overwhelmingly comprehensive information, it condenses the aspects of pharmacology directly relevant to everyday practice into a concise, accessible volume, including material on the half life of drugs, patient non-compliance and severe chronic inflammation.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access How Drugs Work by Hugh McGavock in PDF and/or ePUB format, as well as other popular books in Medicine & Internal Medicine & Diagnosis. We have over one million books available in our catalogue for you to explore.
Information
1 Getting a drug into the body: absorption
It may seem a truism to state that unless a drug is absorbed into the body in sufficient amounts, it will not work.
However, prescribers are often unaware that the drug industry spends perhaps a quarter of its research budget for a new drug on pharmaceutics, i.e. devising the right presentation to ensure that the drug is effectively absorbed, properly distributed, and remains at its site of action long enough to produce an effect. This is often a major problem whose solution we clinicians take for granted, but it may have involved intense research activity and many millions of pounds of research investment.
Of course, this process is by no means a recent development. Over the past 45 years, the following advances have been made:
- capsules and enteric coatings (EC), which avoid, for example, degradation by gastric acid
- modified-release (MR) tablets, which extend the duration of action of the drug
- inactive pro-drugs, which the body's metabolic processes convert to active compounds
- skin patches for transdermal drug delivery
- subcutaneous implants for long-term treatment
- sophisticated inhalers
- drug-releasing vaginal rings and intrauterine devices (IUDs) as an effective means of long-term drug delivery in women
- dosage-adjustable self-injection devices, particularly for insulin-dependent diabetics.
Absorption processes
This chapter describes the five processes that feature in drug absorption:
- passive diffusion down a concentration gradient (most drugs)
- the cell membrane and fat-solubility of drugs (most drugs)
- active transport (some drugs)
- disintegration and dissolution of tablets (many drugs)
- presystemic metabolism (first-pass metabolism) (most drugs).
Passive diffusion down a concentration gradient
Only intravenous and inhaled anti-asthma drugs avoid the need for absorption across cell membranes. Most other drugs are absorbed from the intestine, skin or mucous membranes, mainly by passive diffusion across cell membranes from an area of high drug concentration to one of low concentration, until the concentrations in the two areas are in balance (equilibrium). They reach the blood capillaries by similar passive diffusion, and are distributed around the body.
The rate of absorption of a drug depends on three things: the concentration gradient, the surface area available for absorption and the fat-solubility of the drug itself.
The principle of the concentration gradient is the reason why oral drugs are best absorbed if given well before a meal, as this maximises their concentration in the small intestine, from which most oral drugs are absorbed. Note the very large surface area of the jejunal villi, which makes the jejunum an ideal site for the absorption of the majority of drugs.
The cell membrane and fat-solubility of drugs
Although the cells lining many blood capillaries, particularly the kidney glomerulus, have pores between them allowing relatively free passage of drug molecules, most other tissues, including the intestine, have few intercellular pores large enough to permit drug absorption.
To be absorbed, oral drugs must therefore cross the cell membranes of the intestinal villi. As in all cells, the membrane is composed of a double 'fatty' layer of phospholipid, arranged like a palisade (see Figure 1.1). Although this is a pictorial representation, it is close to the molecular reality revealed by the electron microscope. In essence, it means that there is a well-sealed fatty barrier between the intracellular and extracellular fluids.
Fat-soluble molecules, including some drugs, can pass directly through the cell membrane. But on the right of Figure 1.1, you see that ionised and water-soluble molecules and ions, including some drugs, cannot cross the cell membrane.
Without the innate property of phospholipids to form such membranes, cell life would be impossible, since cellular metabolism depends to a very large extent on the maintenance of strict intracellular control of water and ions such as sodium, potassium and calcium, chloride and bicarbonate, which require active transport into and out of the cell.
An excellent example of passive diffusion and fat solubility is the almost instantaneous absorption of the anti-anginal drug glyceryl trinitrate (GTN) across the buccal (mouth) mucosa. A GTN spray delivers a high concentration of this very lipid-soluble compound of very low molecular weight, which is absorbed almost as fast as an intravenous injection.
Drugs are usually formulated to make them as lipid-soluble as possible, and the pharmaceutical industry has produced a variety of chemical means of achieving this end.
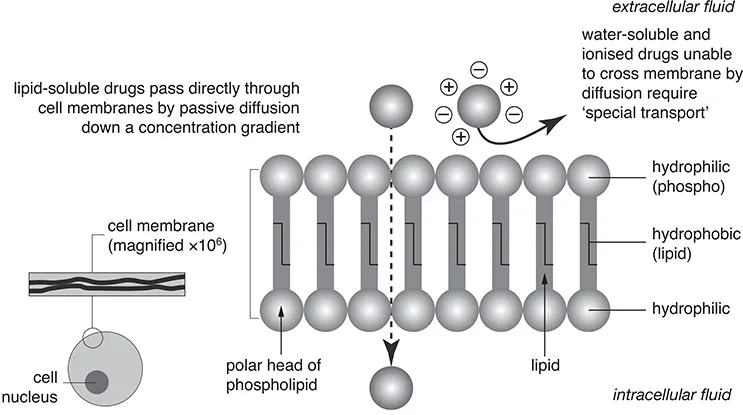
Figure 1.1 How drugs cross the cell membrane.
However, sometimes a drug's low lipid solubility is used to good effect, for example, with the use of aminosalicylates like mesalazine in the treatment of ulcerative colitis, and the antibiotics vancomycin and neomycin. In these examples, the aim is to get the drug into the lumen of the colon for therapeutic purposes, while avoiding systemic absorption.
Many drugs are weak acids or bases (in ionic equilibrium, part ionised, part unionised). In such cases, only the un-ionised form is sufficiently lipid-soluble to diffuse across phospholipid membranes.
Active transport across the intestinal mucosa
All cells have active transport mechanisms. These are essential for carrying ions, most sugars, and the amino acids into and out of the cell in a highly regulated fashion, which will be described later (see Chapter 9).
Active transport is not a very important means of drug absorption, although iron salts, levodopa for Parkinson's disease, the antithyroid drug propylthiouracil and the anticancer drug fluorouracil are actively transported across the intestinal mucosa.
However, it is important to realise at this stage that active transport exists, if only because many of our most effective renal, gastric and other drugs act by increasing or decreasing such cellular transport mechanisms. This is what happens every time we prescribe a proton pump inhibitor, for example to suppress the synthesis of gastric acid.
The importance of disintegration and dissolution of tablets in the stomach
Plain tablets first disintegrate and then dissolve in the stomach. Figure 1.2 shows the way in which a meal delays gastric emptying and, consequently, delays the absorption
of any drug taken during or after food, and the effect that this may have on drug plasma concentration. So, to achieve maximal concentration in the small intestine, where most drugs are absorbed, a drug should be taken before food. This is a simple principle about which we sometimes need reminding, particularly when prescribing most antibiotics, where achieving an adequate tissue concentration is always paramount.
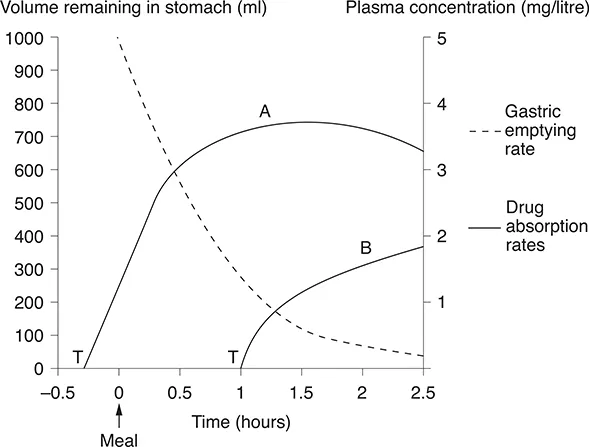
Figure 1.2 A tablet taken before a meal will dissolve in the stomach and enter the small intestine within 15 minutes (curve A); taken during or after a meal, the drug may not reach the intestine for 1-2 hours (curve B). T, time at which tablet is swallowed.
In contrast, where peak plasma concentrations may be associated with side-effects, drugs can be given with food to reduce peaks.
Many modern drugs are formulated to avoid early release; for example, an enteric coating is applied to the tablet when a drug is irritant to the gastric mucosa or when it is rendered inactive by gastric acid.
Modified-release (MR or LA) drugs are used when the duration of action of a short-acting drug needs to be prolonged, as with the heart drug nifedipine. These formulations may also be used when a gradual rise in plasma concentration is desirable, or for patient convenience, in the hope of improving compliance, if the patient has to take the drug less often.
It is essential to remember that when a branded MR product is selected for long-term therapy, as in the treatment of hypertension for example, that that branded version should always be repeated, because different MR brands of the same drug may have clinically important variations in absorption and plasma concentration. The ten MR nifedipine preparations cited in the British National Formulary (BNF) are a good example of this, in treating hypertension.
Among the commonest of self-medications are the antacids. If an antacid is consumed at the same time as certain oral drugs, a chemical reaction occurs between the drug and the antacid, rendering the drug ineffective and leading to treatment failure. This problem is considered in detail in Chapter 26.
Presystemic metabolism
Presystemic metabolism (breakdown of a proportion of a drug by specialised cellular enzymes) takes place before the drug reaches the systemic circulation. It occurs mainly in the liver, but a degree of breakdown also occurs in the intestinal mucosa (see below), lungs and skeletal muscle (see Figure 1.3).
Clearly, extensive metabolism results in a greatly decreased plasma concentration of drug. For example, only one-twentieth of the dose of levodopa survives first-pass metabolism in the liver, and a similar story exists for many drugs.
Practitioners rarely have to consider this factor, since drug dosage is designed to take into account such wastage. This explains the practical importance of bioavailability, which is the fraction of an oral dose that reaches the systemic circulation. Chapters 3 and 4 describe the chemical processes involved in drug metabolism.
Inactivation of drugs...
Table of contents
- Cover
- Title Page
- Copyright Page
- Table of Contents
- Preface to the fourth edition
- Acknowledgments
- Dedication Page
- Chapter 1 Getting a drug into the body: absorption
- Chapter 2 Getting a drug to its site of action: distribution
- Chapter 3 Inactivating drugs: phase 1 drug metabolism
- Chapter 4 Phase 2 drug metabolism and methods of excretion
- Chapter 5 The concept of a drug's half-life (£1/2): an estimate of the rate of elimination of different drugs
- Chapter 6 Receptor function and intercellular signalling
- Chapter 7 The central role of receptors in drug action
- Chapter 8 Drugs that block enzymes: understanding NSAID therapy in inflammation
- Chapter 9 The principal targets for drug action
- Chapter 10 Calcium ion for the prescriber
- DRUGS AND THE CENTRAL NERVOUS SYSTEM
- DRUG TOPICS OF SPECIAL IMPORTANCE
- Conclusion
- Prescribing safely: a checklist for every prescription
- Index